The Intestinal Efflux Transporter Inhibition Activity of Xanthones from Mangosteen Pericarp: An In Silico, In Vitro and Ex Vivo Approach
Abstract
:1. Introduction
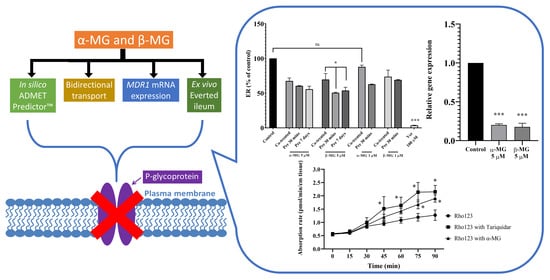
2. Results
2.1. In Silico Study via ADMET PredictorTM Software
2.2. P-Glycoprotein (Pgp) ATPase Assay
2.3. Cell Culture
2.3.1. In Vitro Cytotoxicity Studies
2.3.2. Transport Study
Paracellular Transport Study
Bidirectional Transport Study
2.3.3. MDR1 Expression Analysis Using Real-Time RT-qPCR
2.4. Ex Vivo Absorptive Transport Study across Everted Mouse Ileum
3. Discussion
4. Materials and Methods
4.1. Source of Extracts
4.2. In Silico Study via ADMET PredictorTM Software
4.3. Pgp ATPase Assay
4.4. Cell Culture
4.4.1. In Vitro Cytotoxicity Studies
4.4.2. Transport Study
Paracellular Transport Study
Bidirectional Transport Study
Data Analysis
4.4.3. MDR1 Expression Using a Real-Time Reverse Transcription Quantitative Polymerase Chain Reaction (Real Time RT-qPCR)
4.5. Ex Vivo Absorptive Transport Study across Everted Mouse Ileum
4.6. Statistical Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 23, 1047–1065. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio-Pérez, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Benatrehina, P.A.; Pan, L.; Naman, C.B.; Li, J.; Kinghorn, A.D. Usage, biological activity, and safety of selected botanical dietary supplements consumed in the United States. J. Tradit. Complement. Med. 2018, 8, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, G.; Ling, V. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 1994, 13, 223–233. [Google Scholar] [CrossRef]
- Kwon, H.; Lionberger, R.A.; Yu, L.X. Impact of P-Glycoprotein-Mediated Intestinal Efflux Kinetics on Oral Bioavailability of P-Glycoprotein Substrates. Mol. Pharm. 2004, 1, 455–465. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.-R.; Niu, T.; Gao, S.; Yin, T.; You, M.; Jiang, Z.-H.; Hu, M. Inhibition of P-Glycoprotein Leads to Improved Oral Bioavailability of Compound K, an Anticancer Metabolite of Red Ginseng Extract Produced by Gut Microflora. Drug Metab. Dispos. 2012, 40, 1538–1544. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Chen, L.; Tian, S.; Sun, H.; Hou, T. ADMET Evaluation in Drug Discovery. 13. Development of in Silico Prediction Models for P-Glycoprotein Substrates. Mol. Pharm. 2014, 11, 716–726. [Google Scholar] [CrossRef]
- Hunter, J.; Jepson, M.A.; Tsuruo, T.; Simmons, N.L.; Hirst, B.H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. J. Biol. Chem. 1993, 298, 14991–14997. [Google Scholar]
- Matsumoto, T.; Kaifuchi, N.; Mizuhara, Y.; Warabi, E.; Watanabe, J. Use of a Caco-2 permeability assay to evaluate the efects of several Kampo medicines on the drug transporter P-glycoprotein. J. Nat. Med. 2018, 72, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreño-Gómez, B.; Duncan, R. Everted rat intestinal sacs: A newmodel for the quantitation of P-glycoprotein mediated-efflux ofanticancer agents. Anticancer. Res. 2000, 20, 3157–3161. [Google Scholar] [PubMed]
- Alam, M.A.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Everted gut sac model as a tool in pharmaceutical research: Limitations and applications. J. Pharm. Pharmacol. 2012, 64, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Waller, C.L.; Swaan, P.W.; Cruciani, G.; Wrighton, S.A.; Wikel, J.H. Progress in predicting human ADME parameters in silico. J. Pharmacol. Toxicol. Methods 2000, 44, 251–272. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Yu, H.; Zhang, L.; Hou, T. Computational models for predicting substrates or inhibitors of P-glycoprotein. Drug Discov. Today 2012, 17, 343–351. [Google Scholar] [CrossRef]
- Gleeson, M.P. Generation of a Set of Simple, Interpretable ADMET Rules of Thumb. J. Med. Chem. 2008, 51, 817–834. [Google Scholar] [CrossRef]
- Ekins, S.; Polli, J.E.; Swaan, P.W.; Wright, S.H. Computational modeling to accelerate the identification of substrates and inhibitors for transporters that affect drug disposition. Clin. Pharmacol. Ther. 2012, 92, 661–665. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.A.; Pate, D.W.; Clark, R.D.; Davies, N.M.; El-Kadi, A.O.; Löbenberg, R. Phytocannabinoid drug-drug interactions and their clinical implications. Pharmacol. Ther. 2020, 215, 107621. [Google Scholar] [CrossRef]
- Sousa, E.; Palmeira, A.; Cordeiro, A.S.; Sarmento, B.; Ferreira, D.; Lima, R.T.; Vasconcelos, M.H.; Pinto, M.M.M.; Sousa, M.E. Bioactive xanthones with effect on P-glycoprotein and prediction of intestinal absorption. Med. Chem. Res. 2012, 22, 2115–2123. [Google Scholar] [CrossRef]
- Chae, S.W.; Woo, S.; Park, J.H.; Kwon, Y.; Na, Y.; Lee, H.J. Xanthone analogues as potent modulators of intestinal P-glycoprotein. Eur. J. Med. Chem. 2015, 93, 237–245. [Google Scholar] [CrossRef]
- Martins, E.; Silva, V.; Lemos, A.; Palmeira, A.; Puthongking, P.; De Sousa, M.E.; Rocha-Pereira, C.; Ghanem, C.I.; Carmo, H.; Remião, F.; et al. Newly Synthesized Oxygenated Xanthones as Potential P-Glycoprotein Activators: In Vitro, Ex Vivo, and In Silico Studies. Molecules 2019, 24, 707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laksmiani, N.P.L. Ethanolic extract of mangosteen (Garcinia mangostana) pericarp as sensitivity enhancer of doxorubicin on MCF-7 cells by inhibiting P-glycoprotein. Nusant. Biosci. 2019, 11, 49–55. [Google Scholar] [CrossRef]
- Ledwitch, K.V.; Gibbs, M.E.; Barnes, R.W.; Roberts, A.G. Cooperativity between verapamil and ATP bound to the efflux transporter P-glycoprotein. Biochem. Pharmacol. 2016, 118, 96–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, K.; Strab, R.; Hidalgo, I.J. Cultured Epithelial Cell Assays Used to Estimate Intestinal Absorption Potential. In Pharmaceutical Profiling in Drug Discovery for Lead Selection; Borchardt, R., Kerns, E., Lipinski, C., Thakker, D., Wang, B., Eds.; AAPS Press: Arlington, TX, USA, 2004; pp. 217–234. [Google Scholar]
- Furukawa, K.-I.; Shibusawa, K.; Chairungsrilerd, N.; Ohta, T.; Nozoe, S.; Ohizumi, Y. The Mode of Inhibitory Action of alpha-Mangostin, a Novel Inhibitor, on the Sarcoplasmic Reticulum Ca(2+)-Pum** ATPase from Rabbit Skeletal Muscle. Jpn. J. Pharmacol. 1996, 71, 337–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edward, H.; Kerns, L.D. In Vitro Transporter Methods. In Drug-Like Properties: Concepts, Structure Design and Methods: From ADME to Toxicity Optimization; Edward, H., Kerns, L.D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 300–302. [Google Scholar]
- Chitchumroonchokchai, C.; Riedl, K.M.; Suksumrarn, S.; Clinton, S.K.; Kinghorn, A.D.; Failla, M.L. Xanthones in Mangosteen Juice Are Absorbed and Partially Conjugated by Healthy Adults. J. Nutr. 2012, 142, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez-Orozco, F.; Chitchumroonchokchai, C.; Lesinski, G.B.; Suksamrarn, S.; Failla, M.L. Alpha-Mangostin: Anti-Inflammatory Activity and Metabolism by Human Cells. J. Agric. Food Chem. 2013, 61, 3891–3900. [Google Scholar] [CrossRef] [Green Version]
- Matzneller, P.; Kussmann, M.; Eberl, S.; Maier-Salamon, A.; Jäger, W.; Bauer, M.; Langer, O.; Zeitlinger, M.; Poeppl, W. Pharmacokinetics of the P-gp Inhibitor Tariquidar in Rats After Intravenous, Oral, and Intraperitoneal Administration. Eur. J. Drug Metab. Pharm. 2018, 43, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Brunner, I.; Han, A.-R.; Hamburger, M.; Kinghorn, A.D.; Frye, R.; Butterweck, V. Pharmacokinetics of α-mangostin in rats after intravenous and oral application. Mol. Nutr. Food Res. 2011, 55, S67–S74. [Google Scholar] [CrossRef]
- Boonnak, N.; Chantrapromma, S.; Sathirakul, K.; Kaewpiboon, C. Modified tetra-oxygenated xanthones analogues as anti-MRSA and P. aeruginosa agent and their synergism with vancomycin. Bioorganic Med. Chem. Lett. 2020, 30, 127494. [Google Scholar] [CrossRef]
- Erić, S.; Kalinić, M.; Ilić, K.; Zloh, M. Computational classification models for predicting the interaction of drugs with P-glycoprotein and breast cancer resistance protein. SAR QSAR Environ. Res. 2014, 25, 939–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bachmeier, C.; Miller, D.W. In vitro and in vivo models for assessing drug efflux transporter activity. Adv. Drug Deliv. Rev. 2003, 55, 31–51. [Google Scholar] [CrossRef]
- Didziapetris, R.; Japertas, P.; Avdeef, A.; Petrauskas, A. Classification Analysis of P-Glycoprotein Substrate Specificity. J. Drug Target. 2003, 11, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Broccatelli, F.; Carosati, E.; Neri, A.; Frosini, M.; Goracci, L.; Oprea, T.I.; Cruciani, G. A Novel Approach for Predicting P-Glycoprotein (ABCB1) Inhibition Using Molecular Interaction Fields. J. Med. Chem. 2011, 54, 1740–1751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pgp-Glo™ Assay System: Instruction for Use of Products V3591 and V3601. Madison: Promega Corporation. 2015. Available online: www.promega.com (accessed on 20 January 2014).
- Tomaru, A.; Morimoto, N.; Morishita, M.; Takayama, K.; Fujita, T.; Maeda, K.; Kusuhara, H.; Sugiyama, Y. Studies on the intestinal absorption characteristics of sulfasalazine, a breast cancer resistance protein (BCRP) substrate. Drug Metab. Pharm. 2012, 28, 71–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Properties | α-MG | β-MG |
|---|---|---|
| Likelihood of Pgp Inhibitor (%confidence score) | Yes (97%) | Yes (97%) |
| Likelihood of Pgp Substrate (%confidence score) | Yes (68%) | Yes (95%) |
| Primer | Gene Sequences | |
|---|---|---|
| MDR1 | Forward Reverse | 5′TGC TCA GAC AGG ATG TGA GTT G 3′ 5′AAT TAC AGC AAG CCT GGA ACC 3′ |
| GAPDH | Forward Reverse | 5′GGC CTC CAA GGA GTA AGA CC 3′ 5′AGG GGA GAT TCA GTG TGG TG 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dechwongya, P.; Limpisood, S.; Boonnak, N.; Mangmool, S.; Takeda-Morishita, M.; Kulsirirat, T.; Rukthong, P.; Sathirakul, K. The Intestinal Efflux Transporter Inhibition Activity of Xanthones from Mangosteen Pericarp: An In Silico, In Vitro and Ex Vivo Approach. Molecules 2020, 25, 5877. https://doi.org/10.3390/molecules25245877
Dechwongya P, Limpisood S, Boonnak N, Mangmool S, Takeda-Morishita M, Kulsirirat T, Rukthong P, Sathirakul K. The Intestinal Efflux Transporter Inhibition Activity of Xanthones from Mangosteen Pericarp: An In Silico, In Vitro and Ex Vivo Approach. Molecules. 2020; 25(24):5877. https://doi.org/10.3390/molecules25245877
Chicago/Turabian StyleDechwongya, Panudda, Songpol Limpisood, Nawong Boonnak, Supachoke Mangmool, Mariko Takeda-Morishita, Thitianan Kulsirirat, Pattarawit Rukthong, and Korbtham Sathirakul. 2020. "The Intestinal Efflux Transporter Inhibition Activity of Xanthones from Mangosteen Pericarp: An In Silico, In Vitro and Ex Vivo Approach" Molecules 25, no. 24: 5877. https://doi.org/10.3390/molecules25245877