Polydopamine-Lysophosphatidate-Functionalised Titanium: A Novel Hybrid Surface Finish for Bone Regenerative Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Maintenance of Human Osteoblasts
2.3. Cell Seeding and Treatment at Control and Functionalised Titanium (Ti)
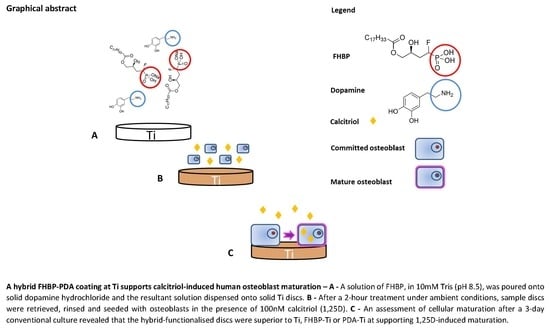
2.4. Functionalisation of Titanium (Ti) Using PDA and FHBP
2.5. Biochemical Detection of PDA at Ti Surfaces
2.6. An Assessment of PDA-FHBP Coating Stability to a Simulated in vivo Environment
2.7. Surface Wettability Measurements
2.8. Raman Spectroscopy
2.9. X-ray Photoelectron Spectroscopy (XPS)
2.10. Preparation of Osteoblast Monolayers in Multi-well Plates for FHBP-PDA Stability Studies
2.11. Metabolic Profile of MG63 Osteoblasts in Receipt of FHBP and 1,25D
2.12. Assessment of Osteoblast Growth at PDA-FHBP-Functionalised Surfaces
2.13. Evaluation of Osteoblast Maturation at PDA-FHBP-Functionalised Surfaces
2.14. Statistical Analysis
3. Results
3.1. Short-Term Development of PDA Films are Compatible with Osteoblast Viability
3.2. PDA Thin Films are Reliably Detected at Ti Surfaces Using a BCA Assay Reagent
3.3. Physicochemical Evidence of PDA Deposition at Ti
3.4. Evidence that FHBP Links to the PDA Matrix via a Schiff-Base Reaction
3.5. PDA-FHBP Hybrid Ti Coatings Support a Good Human Osteoblast Maturation Response
3.6. 1,25D and FHBP Drive a Change in the Metabolic Profile of MG63 Cells
3.7. PDA-FHBP Hybrid Ti Coatings Exhibit Good Stability to a Simulated in vivo Environment
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement-a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Cherian, J.J.; Jauregui, J.J.; Banerjee, S.; Pierce, T.; Mont, M.A. What host factors affect aseptic loosening after THA and TKA? Clin. Orthop. Relat. Res. 2015, 473, 2700–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoghi, P.; Liebensteiner, M.; Agreiter, M.; Leithner, A.; Bohler, N.; Labek, G. Revision surgery after total joint arthroplasty: A complication-based analysis using worldwide arthroplasty registers. J. Arthroplast. 2013, 28, 1329–1332. [Google Scholar] [CrossRef] [PubMed]
- Schroer, W.C.; Berend, K.R.; Lombardi, A.V.; Barnes, C.L.; Bolognesi, M.P.; Berend, M.E.; Ritter, M.A.; Nunley, R.M. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J. Arthroplast. 2013, 28, 116–119. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Lucaciu, G.D.O.; Crisan, B.; Crisan, L.; Baciut, M.; Onisor, F.; Baciut, G.; Câmpian, R.S.; Bran, S. Systemic drugs that influence titanium implant osseointegration. Drug Metab. Rev. 2017, 49, 92–104. [Google Scholar] [CrossRef]
- Mansell, J.P.; Brown, J.; Knapp, J.; Faul, C.F.J.; Blom, A.W. Lysophosphatidic acid-functionalised titanium as a superior surface for supporting human osteoblast (MG63) maturation. Eur. Cells Mater. 2012, 23, 348–361. [Google Scholar] [CrossRef]
- Ayre, W.N.; Scott, T.; Hallam, K.; Blom, A.W.; Denyer, S.; Bone, H.K.; Mansell, J.P. Fluorophosphonate-functionalised titanium via a pre-adsorbed alkane phosphonic acid: A novel dual action surface finish for bone regenerative applications. J. Mater. Sci: Mater. Med. 2016, 27, 36. [Google Scholar] [CrossRef]
- Lancaster, S.Y.; Blackburn, J.; Blom, A.; Makishima, M.; Ishizawa, M.; Mansell, J.P. 24,25 Dihydroxyvitamin D3 cooperates with a stable, fluoromethylene LPA receptor agonist to secure human (MG63) osteoblast maturation. Steroids 2014, 83, 52–61. [Google Scholar] [CrossRef]
- Shiel, A.I.; Ayre, W.N.; Blom, A.W.; Hallam, K.; Heard, P.J.; Payton, O.; Picco, L.; Mansell, J.P. Development of a facile fluorophosphonate-functionalised titanium surface for potential orthopaedic applications. J. Orthop. Trans. 2020. (Accepted for publication). [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired catecholic chemistry for surface modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Maier, G.P.; Butler, A. Siderophores and mussel foot proteins: The role of catechol, cations, and metal coordination in surface adhesion. J. Biol. Inorg. Chem. 2017, 22, 739–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. Acs Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tokura, Y.; Harvey, S.; Chen, C.; Wu, Y.; Ng, D.Y.W.; Weil, T. Fabrication of Defined Polydopamine Nanostructures by DNA Origami-Templated Polymerization. Angew. Chem. Int. Ed. 2018, 57, 1587–1591. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Zhou, J.; **ong, Q.; Ma, J.; Ren, J.; Messersmith, P.B.; Chen, P.; Duan, H. Polydopamine-Enabled Approach toward Tailored Plasmonic Nanogapped Nanoparticles: From Nanogap Engineering to Multifunctionality. Acs Nano 2016, 10, 11066–11075. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl Acad Sci Usa 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [Green Version]
- Lynge, M.E.; Ogaki, R.; Laursen, A.O.; Lovmand, J.; Sutherland, D.S.; Stadler, B. Polydopamine/liposome coatings and their interaction with myoblast cells. Acs Appl Mater. Interfaces 2011, 3, 2142–2147. [Google Scholar] [CrossRef]
- Nirasay, S.; Badia, A.; Leclair, G.; Claverie, J.P.; Marcotte, I. Polydopamine-supported lipid bilayers. Materials 2012, 5, 2621–2636. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.; Chechetka, S.A.; Masuda, M.; Shimizu, T.; Aoyagi, M.; Minamikawa, H.; Miyako, E. Lipid nanotube tailored fabrication of uniquely shaped polydopamine nanofibers as photothermal converters. Chem EurJ. 2016, 22, 4345–4350. [Google Scholar] [CrossRef]
- Clover, J.; Gowen, M. Are MG-63 and HOS TE85 human osteosarcoma cell lines representative models of the osteoblastic phenotype? Bone 1994, 15, 585–591. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast model for in vitro research. Eur Cells Mater 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Gidley, J.; Openshaw, S.; Pring, E.T.; Sale, S.; Mansell, J.P. Lysophosphatidic acid cooperates with 1α,25(OH)2D3 in stimulating human MG63 osteoblast maturation. Prost. Other Lipid Med. 2006, 80, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Mansell, J.P.; Cooke, M.; Read, M.; Rudd, H.; Shiel, A.I.; Wilkins, K.; Manso, M. Chitinase 3-like 1 expression by human (MG63) osteoblasts in response to lysophosphatidic acid and 1,25-dihydroxyvitamin D3. Biochimie 2016, 128-129, 193–200. [Google Scholar] [CrossRef]
- Neary, G.; Blom, A.W.; Shiel, A.I.; Wheway, G.; Mansell, J.P. Development and biological evaluation of fluorophosphonate-modified hydroxyapatite for orthopaedic applications. J. Mater. Sci: Mater. Med. 2019, 30, 126. [Google Scholar] [CrossRef] [Green Version]
- Andrea, A.; Mansell, J.P. A Facile and sensitive colorimetric approach to confirming the presence of polydopamine thin films on (bio)material surfaces. Regen Med.. 2018, 2, 30–36. [Google Scholar] [CrossRef]
- Kwon, I.S.; Bettinger, C.J. Polydopamine nanostructures as biomaterials for medical applications. J. Mater. Chem B 2018, 6, 6895–6903. [Google Scholar] [CrossRef]
- Steeves, A.J.; Variola, F. Elucidating structure-function relationships governing the interfacial response of human mesenchymal stem cells to polydopamine coatings. J. Mater. Chem B 2020, 8, 199–215. [Google Scholar] [CrossRef]
- Malollari, K.G.; Delparastan, P.; Sobek, C.; Vachhani, S.J.; Fink, T.D.; Zha, R.H.; Messersmith, P.B. Mechanical enhancement of bioinspired polydopamine nanocoatings. Acs Appl Mater. Interfaces 2019, 11, 43599–43607. [Google Scholar] [CrossRef]
- Liebscher, J.; Mrowczynski, R.; Scheidt, H.A.; Filip, C.; Hadade, N.D.; Turcu, R.; Bende, A.; Beck, S. Structure of polydopamine: A never-ending story? Langmuir 2013, 29, 10539–10548. [Google Scholar] [CrossRef]
- Cliff, T.S.; Dalton, S. Metabolic switching and cell fate decisions: Implications for pluripotency, reprogramming and development. Curr Opin Genet. Dev. 2017, 46, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.F.; Arruda, I.R.S.; Silva, G.M.M.; Machado, M.; Coelho, L.C.B.B.; Correia, M.T.S. Functionalization of titanium dioxide nanotubes with biomolecules for biomedical applications. Mat. Sci Eng C 2017, 81, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Whyte, M.P. Physiological role of alkaline phosphatase explored in hypophosphatasia. Ann. Ny Acad Sci 2010, 1192, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Paz, Y. Self-assembled monolayers and titanium dioxide: From surface patterning to potential applications. BeilsteinJ. Nanotech 2011, 2, 845–861. [Google Scholar] [CrossRef] [Green Version]
- Queffélec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface modification using phosphonic ccids and esters. Chem Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Guangchun, J.; Jiyeon, K.; Lingmin, Y.U.; Wookang, Y.; Myung**, L.; Ilsong, P.K.; Minho, L.; Dongchun, J. Surface characteristics of mussel-inspired polydopamine coating on titanium substrates. J. Wuhan Uni Tech.-Mater. Sci Ed. 2014, 29, 197–200. [Google Scholar]
- Xu, D.; Yang, W.; Hu, Y.; Luo, Z.; Li, J.; Hou, Y. Surface functionalization of titanium substrates with cecropin B to improve their cytocompatibility and reduce inflammation responses. Coll Surf. B: Biointerf 2013, 110, 225–235. [Google Scholar] [CrossRef]
- Ye, J.; Shao, C.; Zhang, X.; Guo, X.; Gao, P.; Cen, Y.; Ma, S.; Liu, Y. Effects of DNase I coating of titanium on bacteria adhesion and biofilm formation. Mat. Sci Eng C 2017, 78, 738–747. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid mussel-nspired surface zwitteration for enhanced antifouling and antibacterial properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Jiang, H.; Ren, D.; Wang, H.; Hu, Y.; Guo, S.; Yuan, H.; Hu, P.; Zhang, L.; Li, C. 2D Monolayer MoS 2 –carbon interoverlapped superstructure: Engineering ideal atomic interface for lithium ion storage. Adv. Mater 2015, 27, 3687–3695. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Z.; Wang, Y.; Dong, Y.; Liu, Y.; Wang, X.; Qiu, J. Carbon-stabilized interlayer-expanded few-layer MoSe2 nanosheets for sodium ion batteries with enhanced rate capability and cycling performance. Acs Appl Mater. Interf 2016, 8, 32324–32332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-P.; Liu, Y.-P.; Yuan, Z.-Z. Biochemistry-inspired direct synthesis of nitrogen and phosphorus dual-doped microporous carbon spheres for enhanced electrocatalysis. Chem Commun 2016, 52, 2118–2121. [Google Scholar] [CrossRef]
- Jia, L.; Han, F.; Wang, H.; Zhu, C.; Guo, Q.; Li, J.; Zhao, Z.; Zhang, Q.; Zhu, X.; Li, B. Polydopamine-assisted surface modification for orthopaedic implant. J. Orthop Trans. 2019, 17, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Yimsiri, P.; Mackley, M.R. Spin and dip coating of light-emitting polymer solutions: Matching experiment with modelling. Chem Eng Sci 2006, 61, 3496–3505. [Google Scholar] [CrossRef]
- Fu, L.; Yu, A.M. Carbon nanotubes based thin films: Fabrication, characterization and applications. Rev. Adv. Mater. Sci 2014, 36, 40–61. [Google Scholar]
- Chaki, S.H.; Mahato, K.S.; Malek, T.J.; Deshpande, M.P. CuAlS2 thin films-dip coating deposition and characterization. J. Sci Adv. Mater. Devices 2017, 2, 215–224. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







| Surface Modification | Blank Ti | FHBP-Ti | PDA-Ti | FHBP-PDA-Ti |
|---|---|---|---|---|
| Contact angle | 66.7 ± 5.1° | 65.3 ± 2.8° | 54.4 ± 3.9° * | 55.7 ± 5.8° * |
| PDA coating-μg/mL DHC equivalents | 2.1 ± 1.04 | 2.37 ± 0.87 |
| Sample | C1s (at. %) | N1s (at. %) | O1s (at. %) | P2p (at. %) | Ti2p (at. %) |
|---|---|---|---|---|---|
| Bare | 50 | 1 | 36 | – | 13 |
| PDA | 63 | 5 | 28 | – | 4 |
| Hybrid | 67 | 6 | 23 | 1 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldwin, F.; Craig, T.J.; Shiel, A.I.; Cox, T.; Lee, K.; Mansell, J.P. Polydopamine-Lysophosphatidate-Functionalised Titanium: A Novel Hybrid Surface Finish for Bone Regenerative Applications. Molecules 2020, 25, 1583. https://doi.org/10.3390/molecules25071583
Baldwin F, Craig TJ, Shiel AI, Cox T, Lee K, Mansell JP. Polydopamine-Lysophosphatidate-Functionalised Titanium: A Novel Hybrid Surface Finish for Bone Regenerative Applications. Molecules. 2020; 25(7):1583. https://doi.org/10.3390/molecules25071583
Chicago/Turabian StyleBaldwin, Fiona, Tim J. Craig, Anna I. Shiel, Timothy Cox, Kyueui Lee, and Jason P. Mansell. 2020. "Polydopamine-Lysophosphatidate-Functionalised Titanium: A Novel Hybrid Surface Finish for Bone Regenerative Applications" Molecules 25, no. 7: 1583. https://doi.org/10.3390/molecules25071583