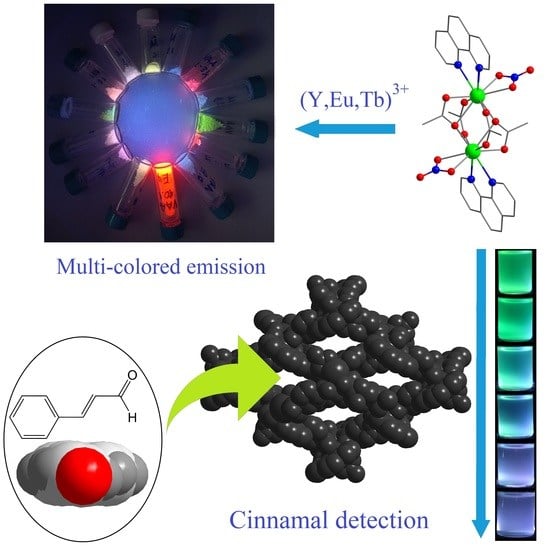
Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Crystal Structure
2.2. Luminescent Properties
2.3. Luminescent Sensing
3. Experimental Section
3.1. Reagents
3.2. Instruments
3.3. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hanna, S.L.; Redfern, L.R.; Alezi, D.; Islamoglu, T.; Farha, O.K. Reticular chemistry in the rational synthesis of functional zirconium cluster-based MOFs. Coord. Chem. Rev. 2019, 386, 32–49. [Google Scholar] [CrossRef]
- Yaghi, O.M. Reticular chemistry in all dimensions. ACS Cent. Sci. 2019, 5, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Ali Akbar Razavi, S.; Morsali, A. Linker functionalized metal-organic frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Deliyanni, E.A. Metal organic frameworks: Synthesis and application. Molecules 2020, 25, 960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, S.L.; Champness, N.R. A periodic table of metal-organic frameworks. Coord. Chem. Rev. 2020, 414, 213295. [Google Scholar] [CrossRef]
- Raptopoulou, C.P. Metal-organic frameworks: Synthetic methods and potential applications. Materials 2021, 14, 310. [Google Scholar] [CrossRef]
- Velasco, E.; Osumi, Y.; Teat, S.J.; Jensen, S.; Tan, K.; Thonhauser, T.; Li, J. fluorescent detection of carbon disulfide by a highly emissive and robust isoreticular series of zr-based luminescent metal organic frameworks (LMOFs). Chemistry 2021, 3, 327–337. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Sapchenko, S.A.; Dybtsev, D.N.; Fedin, V.P. Scandium-organic frameworks: Progress and prospects. Russ. Chem. Rev. 2018, 87, 1139–1167. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef] [PubMed]
- Garai, B.; Bon, V.; Krause, S.; Schwotzer, F.; Gerlach, M.; Senkovska, I.; Kaskel, S. Tunable flexibility and porosity of the metal–organic framework dut-49 through postsynthetic metal exchange. Chem. Mater. 2020, 32, 889–896. [Google Scholar] [CrossRef]
- Demakov, P.A.; Bogomyakov, A.S.; Urlukov, A.S.; Andreeva, A.Y.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Transitio metal coordination polymers with trans-1,4-cyclohexanedicarboxylate: Acidity-controlled synthesis, structures and properties. Materials 2020, 13, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubskikh, V.A.; Lysova, A.A.; Samsonenko, D.G.; Lavrov, A.N.; Kovalenko, K.A.; Dybtsev, D.N.; Fedin, V.P. 3D metal-organic frameworks based on Co(II) and bithiophendicarboxylate: Synthesis, crystal structures, gas adsorption, and magnetic properties. Molecules 2021, 526, 1269. [Google Scholar] [CrossRef] [PubMed]
- Nonat, A.M.; Charbonnière, L. Upconversion of light with molecular and supramolecular lanthanide complexes. Coord. Chem. Rev. 2020, 409, 213192. [Google Scholar] [CrossRef]
- Saraci, F.; Quezada-Novoa, V.; Rafael Donnarumma, P.; Howarth, A.J. Rare-earth metal–organic frameworks: From structure to applications. Chem. Soc. Rev. 2020, 49, 7949–7977. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-P.; Li, D.-S.; ** Effect on Upconversion Emission of NaYF4: Yb3+, Er3+/Tm3+ Microparticles. Materials 2020, 13, 3397. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-W.; Dong, G.; Cui, R.; Li, X. 3D lanthanide-coordination frameworks constructed by a ternary mixed-ligand: Crystal structure, luminescence and luminescence sensing. CrystEngComm 2020, 22, 740–750. [Google Scholar] [CrossRef]
- Gontcharenko, V.E.; Kiskin, M.A.; Dolzhenko, V.D.; Korshunov, V.M.; Taydakov, I.V.; Belousov, Y.A. Mono- and mixed metal complexes of Eu3+, Gd3+, and Tb3+ with a diketone, bearing pyrazole moiety and CHF2-group: Structure, color tuning, and kinetics of energy transfer between lanthanide ions. Molecules 2021, 26, 2655. [Google Scholar] [CrossRef]
- Green, A.P.; Buckley, A.R. Solid state concentration quenching of organic fluorophores in PMMA. Phys. Chem. Chem. Phys. 2015, 17, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.S.; Park, S.-R.; Suh, M.C. Concentration quenching behavior of thermally activated delayed fluorescence in a solid film. J. Phys. Chem. C 2017, 121, 13986–13997. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Kiskin, M.A.; Samsonenko, D.G.; Ryadun, A.A.; Dybtsev, D.N.; Fedin, V.P. Luminescent detection by coordination polymers derived from a pre-organized heterometallic carboxylic building unit. Polyhedron 2018, 145, 147–153. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Zhang, G. A carbazole-functionalized metal–organic framework for efficient detection of antibiotics, pesticides and nitroaromatic compounds. Dalton Trans. 2019, 48, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Sapianik, A.A.; Kiskin, M.A.; Kovalenko, K.A.; Samsonenko, D.G.; Dybtsev, D.N.; Audebrand, N.; Sun, Y.; Fedin, V.P. Rational synthesis and dimensionality tuning of MOFs from preorganized heterometallic molecular complexes. Dalton Trans. 2019, 48, 3676–3686. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.; Wu, S.; Wang, J.; Zhu, M.; Zhang, Y.; Fedin, V.P. Water-stable lanthanide coordination polymers with triple luminescent centers for tunable emission and efficient self-calibration sensing wastewater pollutants. Adv. Opt. Mater. 2020, 8, 1901659. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Matveevskaya, M.; Pavlov, D.; Yakunenkov, A.; Potapov, A. Coordination polymers based on highly emissive ligands: Synthesis and functional properties. Materials 2020, 13, 2699. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, X.; Gao, E.; Wu, S.; Zhang, Y.; Zhu, M. Bifunctional luminescent Eu metal–organic framework for sensing nitroaromatic pollutants and Fe3+ ion with high sensitivity and selectivity. Appl. Organomet. Chem. 2021, 35, e6136. [Google Scholar] [CrossRef]
- Huangfu, M.; Wang, M.; Lin, C.; Wang, J.; Wu, P. Luminescent metal–organic frameworks as chemical sensors based on “mechanism–response”: A review. Dalton Trans. 2021, 50, 3429–3449. [Google Scholar] [CrossRef]
- Svetogorov, R.D.; Dorovatovskii, P.V.; Lazarenko, V.A. Belok/XSA Diffraction beamline for studying crystalline samples at kurchatov synchrotron radiation source. Cryst. Res. Technol. 2020, 55, 1900184. [Google Scholar] [CrossRef]
- Lazarenko, V.A.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Burlov, A.S.; Koshchienko, Y.V.; Vlasenko, V.G.; Khrustalev, V.N. High-throughput small-molecule crystallography at the ‘belok’ beamline of the kurchatov synchrotron radiation source: Transition metal complexes with azomethine ligands as a case study. Crystals 2017, 7, 325. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demakov, P.A.; Vasileva, A.A.; Volynkin, S.S.; Ryadun, A.A.; Samsonenko, D.G.; Fedin, V.P.; Dybtsev, D.N. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules 2021, 26, 5145. https://doi.org/10.3390/molecules26175145
Demakov PA, Vasileva AA, Volynkin SS, Ryadun AA, Samsonenko DG, Fedin VP, Dybtsev DN. Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate. Molecules. 2021; 26(17):5145. https://doi.org/10.3390/molecules26175145
Chicago/Turabian StyleDemakov, Pavel A., Alena A. Vasileva, Sergey S. Volynkin, Alexey A. Ryadun, Denis G. Samsonenko, Vladimir P. Fedin, and Danil N. Dybtsev. 2021. "Cinnamal Sensing and Luminescence Color Tuning in a Series of Rare-Earth Metal−Organic Frameworks with Trans-1,4-cyclohexanedicarboxylate" Molecules 26, no. 17: 5145. https://doi.org/10.3390/molecules26175145