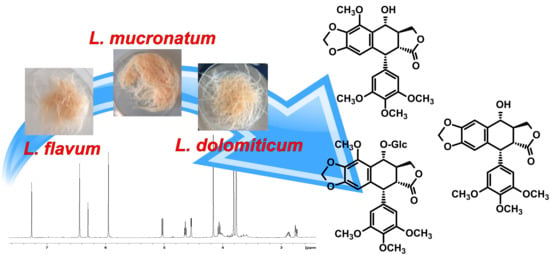
Enhanced Aryltetralin Lignans Production in Linum Adventi-Tious Root Cultures
Abstract
:1. Introduction
2. Results
2.1. ARc Production and Effect of Elicitation Treatments
2.2. Total Phenols, Total Flavonoids Content and DPPH Radical Scavenging Activity
2.3. NMR Identification of ATLs
2.4. Quantitative Analyses of ATLs
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Establishment of ARc and Elicitor Treatments
4.3.1. ARc Induction
4.3.2. Elicitor Treatments
4.3.3. ARc Growth and Viability
4.4. Extraction
4.5. Total Phenols, Total Flavonoids Content and DPPH Radical Scavenging Activity
4.5.1. Total Phenols Content
4.5.2. Total Flavonoids Content
4.5.3. DPPH Radical Scavenging Activity
4.5.4. HPTLC-DPPH Test
4.6. HPLC Analysis
4.7. Preparative TLC, Semi-Preparative HPLC
4.8. NMR Identification of MPTOX and MPTOX–Glc
4.9. Digestion of MPTOX–Glc with β-Glucosidase
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gordaliza, M.; García, P.A.; del Corral, J.M.M.; Castro, M.A.; Gómez-Zurita, M.A. Podophyllotoxin: Distribution, Sources, Applications and New Cytotoxic Derivatives. Toxicon Off. J. Int. Soc. Toxinol. 2004, 44, 441–459. [Google Scholar] [CrossRef] [PubMed]
- Hano, C.; Corbin, C.; Drouet, S.; Quéro, A.; Rombaut, N.; Savoire, R.; Molinié, R.; Thomasset, B.; Mesnard, F.; Lainé, E. The Lignan (+)-Secoisolariciresinol Extracted from Flax Hulls Is an Effective Protectant of Linseed Oil and Its Emulsion against Oxidative Damage. Eur. J. Lipid Sci. Technol. 2017, 119, 1600219. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Shin, D. Arylnaphthalene Lactones: Structures and Pharmacological Potentials. Phytochem. Rev. 2021, 22. [Google Scholar] [CrossRef]
- Cui, Q.; Du, R.; Liu, M.; Rong, L. Lignans and Their Derivatives from Plants as Antivirals. Molecules 2020, 25, 183. [Google Scholar] [CrossRef] [Green Version]
- Nitiss, J.L. Targeting DNA Topoisomerase II in Cancer Chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [Green Version]
- Albertson, A.K.F.; Lumb, J.-P. The Lignans. In Recent Advances in Polyphenol Research; John Wiley & Sons Ltd.: Chichester, UK, 2019; pp. 1–70. ISBN 978-1-119-42789-6. [Google Scholar]
- Suzuki, S.; Umezawa, T. Biosynthesis of Lignans and Norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Yang, L.; Tian, X. Podophyllotoxin: Current Perspectives. Curr. Bioact. Compd. 2007, 3, 37–66. [Google Scholar] [CrossRef]
- Lalaleo, L.; Khojasteh, A.; Fattahi, M.; Bonfill, M.; Cusido, R.M.; Palazon, J. Plant Anti-Cancer Agents and Their Biotechnological Production in Plant Cell Biofactories. Curr. Med. Chem. 2016, 23, 4418–4441. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, M.; Hammerer, L.; Hetmann, M.; Borg, A.; Schmermund, L.; Steiner, L.; Hartmann, P.; Belaj, F.; Kroutil, W.; Gruber, K.; et al. Chemoenzymatic Total Synthesis of Deoxy-, Epi-, and Podophyllotoxin and a Biocatalytic Kinetic Resolution of Dibenzylbutyrolactones. Angew. Chem. Int. Ed. 2019, 58, 8226–8230. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, X.; Renata, H. Asymmetric Chemoenzymatic Synthesis of (−)-Podophyllotoxin and Related Aryltetralin Lignans. Angew. Chem. Int. Ed. 2019, 58, 11657–11660. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.; Xu, H. Recent Advances in the Chemistry and Biology of Podophyllotoxins. Chem. Eur. J. 2017, 23, 4467–4526. [Google Scholar] [CrossRef]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A Novel Potential Natural Anticancer Agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar]
- Muir, A.D.; Westcott, N.D.; Westcott, N.D. Flax: The Genus Linum; CRC Press: Boca Raton, FL, USA, 2003; ISBN 978-0-429-20585-9. [Google Scholar]
- Malik, S.; Bíba, O.; Grúz, J.; Arroo, R.R.J.; Strnad, M. Biotechnological Approaches for Producing Aryltetralin Lignans from Linum Species. Phytochem. Rev. 2014, 13, 893–913. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Hemmati, S.; Klaes, M.; Konuklugil, B.; Mohagheghzadeh, A.; Ionkova, I.; Fuss, E.; Wilhelm Alfermann, A. Lignans in Flowering Aerial Parts of Linum Species—Chemodiversity in the Light of Systematics and Phylogeny. Phytochemistry 2010, 71, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in Seeds of Linum Species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.; Bedorf, N.; Mollenschott, C.; Wray, V.; Sasse, F.; Höfle, G. On the Podophyllotoxins of Root Cultures of Linum flavum. Planta Med. 1988, 54, 204–206. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Namdeo, A. Plant Cell Elicitation for Production of Secondary Metabolites: A Review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Phyton Biotech. Available online: https://phytonbiotech.com/ (accessed on 22 August 2021).
- Fuss, E. Lignans in Plant Cell and Organ Cultures: An Overview. Phytochem. Rev. 2003, 2, 307–320. [Google Scholar] [CrossRef]
- Verpoorte, R.; Memelink, J. Engineering Secondary Metabolite Production in Plants. Curr. Opin. Biotechnol. 2002, 13, 181–187. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Paek, K.-Y. Tools for Biotechnological Production of Useful Phytochemicals from Adventitious Root Cultures. Phytochem. Rev. 2016, 15, 129–145. [Google Scholar] [CrossRef]
- Bektas, Y.; Eulgem, T. Synthetic Plant Defense Elicitors. Front. Plant Sci. 2015, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wichers, H.J.; Versluis-De Haan, G.G.; Marsman, J.W.; Harkes, M.P. Podophyllotoxin Related Lignans in Plants and Cell Cultures of Linum flavum. Phytochemistry 1991, 30, 3601–3604. [Google Scholar] [CrossRef]
- Mohagheghzadeh, A.; Hemmati, S.; Alfermann, A.W. Quantification of Aryltetralin Lignans in Linum album Organs and In Vitro Cultures. Iran. J. Pharm. Sci. 2006, 2, 47–56. [Google Scholar]
- Konuklugil, B.; Schmidt, T.J.; Alfermann, A.W. Accumulation of Lignans in Suspension Cultures of Linum mucronatum ssp. Armenum (Bordz.) Davis. Z. Nat. C J. Biosci. 2001, 56, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Berlin, J.; Wray, V.; Mollenschott, C.; Sasse, F. Formation of Beta-Peltatin-A Methyl Ether and Coniferin by Root Cultures of Linum flavum. J. Nat. Prod. 1986, 49, 435–439. [Google Scholar] [CrossRef]
- Gaosheng, H.; **gming, J. Production of Useful Secondary Metabolites Through Regulation of Biosynthetic Pathway in Cell and Tissue Suspension Culture of Medicinal Plants; IntechOpen: London, UK, 2012; ISBN 978-953-51-0787-3. [Google Scholar]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Lalaleo, L.; Alcazar, R.; Palazon, J.; Moyano, E.; Cusido, R.M.; Bonfill, M. Comparing Aryltetralin Lignan Accumulation Patterns in Four Biotechnological Systems of Linum album. J. Plant. Physiol. 2018, 228, 197–207. [Google Scholar] [CrossRef]
- Ionkova, I. Effect of Methyl Jasmonate on Production of Ariltetralin Lignans in Hairy Root Cultures of Linum tauricum. Pharmacogn. Res. 2009, 1, 102–105. [Google Scholar]
- Sasheva, P.; Ionkova, I.; Stoilova, N. Methyl Jasmonate Induces Enhanced Podophyllotoxin Production in Cell Cultures of Thracian Flax (Linum thracicum ssp. Thracicum). Nat. Prod. Commun. 2015, 10, 1225–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashani, K.; Jalali Javaran, M.; Sabet, M.S.; Moieni, A. Identification of Rate-Limiting Enzymes Involved in Paclitaxel Biosynthesis Pathway Affected by Coronatine and Methyl-β-Cyclodextrin in Taxus baccata L. Cell Suspension Cultures. DARU J. Pharm. Sci. 2018, 26, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) Hairy Root Cultures for the Production of Anticancer Aryltetralin Lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadi, A.; Jafari, M.; Nejhad, N.M.; Hossenian, F. Podophyllotoxin and 6-Methoxy Podophyllotoxin Production in Hairy Root Cultures of Liunm mucronatum ssp. Mucronatum. Pharmacogn. Mag. 2014, 10, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Federolf, K.; Alfermann, A.W.; Fuss, E. Aryltetralin-Lignan Formation in Two Different Cell Suspension Cultures of Linum album: Deoxypodophyllotoxin 6-Hydroxylase, a Key Enzyme for the Formation of 6-Methoxypodophyllotoxin. Phytochemistry 2007, 68, 1397–1406. [Google Scholar] [CrossRef]
- Alejandre-García, I.; Álvarez, L.; Cardoso-Taketa, A.; González-Maya, L.; Antúnez, M.; Salas-Vidal, E.; Díaz, J.F.; Marquina-Bahena, S.; Villarreal, M.L. Cytotoxic Activity and Chemical Composition of the Root Extract from the Mexican Species Linum scabrellum: Mechanism of Action of the Active Compound 6-Methoxypodophyllotoxin. Evid. Based Complement. Altern. Med. ECAM 2015, 2015, 298463. [Google Scholar] [CrossRef] [Green Version]
- Doussot, J.; Mathieu, V.; Colas, C.; Molinie, R.; Corbin, C.; Montguillon, J.; Banuls, L.M.Y.; Renouard, S.; Lamblin, F.; Dupre, P.; et al. Investigation of the Lignan Content in Extracts from Linum, Callitris and Juniperus Species in Relation to Their In Vitro Antiproliferative Activities. Planta Med. 2017, 83, 574–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikac, S.; Markulin, L.; Drouet, S.; Corbin, C.; Tungmunnithum, D.; Kiani, R.; Kabra, A.; Abbasi, B.H.; Renouard, S.; Bhambra, A.; et al. Bioproduction of Anticancer Podophyllotoxin and Related Aryltretralin-Lignans in Hairy Root Cultures of Linum flavum L. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 503–540. ISBN 978-3-030-30185-9. [Google Scholar]
- Mascheretti, I.; Alfieri, M.; Lauria, M.; Locatelli, F.; Consonni, R.; Cusano, E.; Dougué Kentsop, R.A.; Laura, M.; Ottolina, G.; Faoro, F.; et al. New Insight into Justicidin B Pathway and Production in Linum austriacum. Int. J. Mol. Sci. 2021, 22, 2507. [Google Scholar] [CrossRef]
- Rahmat, E.; Kang, Y. Adventitious Root Culture for Secondary Metabolite Production in Medicinal Plants: A Review. J. Plant Biotechnol. 2019, 46, 143–157. [Google Scholar] [CrossRef]
- Choi, S.M.; Son, S.H.; Yun, S.R.; Kwon, O.W.; Seon, J.H.; Paek, K.Y. Pilot-Scale Culture of Adventitious Roots of Ginseng in a Bioreactor System. Plant Cell Tissue Organ. Cult. 2000, 62, 187–193. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Steponkus, P.L.; Lanphear, F.O. Refinement of the Triphenyl Tetrazolium Chloride Method of Determining Cold Injury. Plant. Physiol. 1967, 42, 1423–1426. [Google Scholar] [CrossRef] [Green Version]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin-Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef]
- Jesionek, W.; Majer-Dziedzic, B.; Choma, I.M. Separation, Identification, and Investigation of Antioxidant Ability of Plant Extract Components Using TLC, LC–MS, and TLC–DPPH. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 1147–1153. [Google Scholar] [CrossRef]





| Analytes | Rt (min) | Concentration Range (mg/L) | Regression Equation | Correlation Coefficient (R2) | LOD (mg/L) | LOQ (mg/L) |
|---|---|---|---|---|---|---|
| PTOX | 22.2 | 250–1700 | y = 639956.6x + 625206.9 | 0.9999 | 20.2 | 67.3 |
| MPTOX | 23.9 | 107–533 | y = 509126.1x − 508145.6 | 0.9985 | 23.1 | 76.9 |
| MPTOX―Glc | 18.8 | 125–1000 | y = 334707.7x − 586706.3 | 0.9994 | 32.7 | 109.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.; Mascheretti, I.; Dougué Kentsop, R.A.; Consonni, R.; Locatelli, F.; Mattana, M.; Ottolina, G. Enhanced Aryltetralin Lignans Production in Linum Adventi-Tious Root Cultures. Molecules 2021, 26, 5189. https://doi.org/10.3390/molecules26175189
Alfieri M, Mascheretti I, Dougué Kentsop RA, Consonni R, Locatelli F, Mattana M, Ottolina G. Enhanced Aryltetralin Lignans Production in Linum Adventi-Tious Root Cultures. Molecules. 2021; 26(17):5189. https://doi.org/10.3390/molecules26175189
Chicago/Turabian StyleAlfieri, Michela, Iride Mascheretti, Roméo A. Dougué Kentsop, Roberto Consonni, Franca Locatelli, Monica Mattana, and Gianluca Ottolina. 2021. "Enhanced Aryltetralin Lignans Production in Linum Adventi-Tious Root Cultures" Molecules 26, no. 17: 5189. https://doi.org/10.3390/molecules26175189