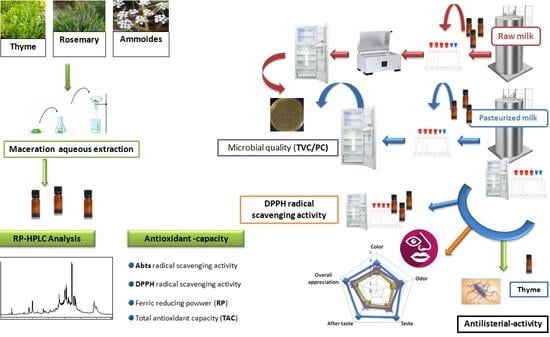
Functionalization of Pasteurized Milk Using Rosemary, Thyme, and Ammoides Aqueous Extracts for Better Microbial Quality and an Improved Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extracts Phytochemicals Contents and Antioxidant Activities
2.2. Extracts Phenolic Profile
2.3. Total Viable and Psychrotrophic Bacteria Count in Milk Supplemented with Plants Extracts
2.3.1. Heat-Treated Supplemented Raw Milk
2.3.2. Cold-Stored Supplemented Pasteurized Milk
2.4. Anti-Listerial Activity in Milk
2.5. Antioxidant Activity of Milk Serum
2.6. Sensory Evaluation
3. Materials and Methods
3.1. Extracts Characterization
3.1.1. Plant Materials and Aqueous Extracts Preparation
3.1.2. Extracts’ Phytochemicals Contents
3.1.3. Antioxidant Activities Evaluation
3.1.4. HPLC analysis of Phenolic Compounds
3.2. Fortified Milk Analysis
3.2.1. Fortified Milk Samples Preparation
3.2.2. Fortified Milk Microbial Quality
3.2.3. Anti-Listerial Activity
3.2.4. Antioxidant Activities of Fortified Milk Serum
3.2.5. Sensory Evaluation
3.3. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groupement International des Viandes Rouges et du Lait GIVLait, Rapport. Available online: http://www.givlait.com.tn (accessed on 3 March 2022).
- Fechner, K.; Dreymann, N.; Schimkowiak, S.; Czerny, C.P.; Teitzel, J. Efficacy of Dairy On-Farm High-Temperature, Short-Time Pasteurization of Milk on the Viability of Mycobacterium avium Ssp. paratuberculosis. J. Dairy Sci. 2019, 102, 11280–11290. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, V.; Velázquez, G.; de Jesús Rodríguez Altaif, R.; Fagotti, F.; Welti-Chanes, J.; Torres, J.A. Deterministic and Probabilistic Predictive Microbiology-Based Indicator of the Listeriosis and Microbial Spoilage Risk of Pasteurized Milk Stored in Residential Refrigerators. LWT 2020, 117, 108650. [Google Scholar] [CrossRef]
- El Ouariachi, E.M.; Tomi, P.; Bouyanzer, A.; Hammouti, B.; Desjobert, J.M.; Costa, J.; Paolini, J. Chemical Composition and Antioxidant Activity of Essential Oils and Solvent Extracts of Ptychotis verticillata from Morocco. Food Chem. Toxicol. 2011, 49, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Boone, L.; Alvarez-Román, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V.M.; de-Torres, N.W.; González, G.; Pérez-López, L.A. Antimicrobial and Antioxidant Activities and Chemical Characterization of Essential Oils of Thymus vulgaris, rosmarinus officinalis, and origanummajorana from Northeastern México. Pak. J. Pharm. Sci. 2015, 28, 363–369. [Google Scholar]
- Laouer, H.; Zerroug, M.M.; Sahli, F.; Chaker, A.N.; Valentini, G.; Ferretti, G.; Grande, M.; Anaya, J. Composition and Antimicrobial Activity of Ammoides pusilla (Brot.) Breistr. Essential Oil. J. Essent. Oil Res. 2003, 15, 135–138. [Google Scholar] [CrossRef]
- Iseppi, R.; Sabia, C.; de Niederhäusern, S.; Pellati, F.; Benvenuti, S.; Tardugno, R.; Bondi, M.; Messi, P. Antibacterial Activity of Rosmarinus officinalis L. and Thymus vulgaris L. Essential Oils and Their Combination against Food-Borne Pathogens and Spoilage Bacteria in Ready-to-Eat Vegetables. Nat. Prod. Res. 2019, 33, 3568–3572. [Google Scholar] [CrossRef]
- Kubatka, P.; Uramova, S.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Liskova, A.; Mojzis, J.; Adamkov, M.; et al. Anticancer Activities of Thymus vulgaris L. In Experimental Breast Carcinoma in Vivo and in Vitro. Int. J. Mol. Sci. 2019, 20, 1749. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadi, T.; Rajabi, S.; Chanda, W.; Sharifi-Rad, M.; et al. Thymus Spp. Plants—Food Applications and Phytopharmacy Properties. Trends Food Sci. Technol. 2019, 85, 287–306. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic Acid—From Bench to Valuable Applications in Food Industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Belkhodja, H.; Belhouala, K.; Nehal, S. Phytochemical Screening and Evaluation of the Antiarthritic Potentialof Ammoides Pusilla Aqueous Extract on Freund’s Adjuvant-Induced Rheumatoid Arthritis. Pharm. Sci. 2020, 27, 170–182. [Google Scholar] [CrossRef]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant Activity and Polyphenol Content of Turkish Thyme (Thymus vulgaris) Monitored by Liquid Chromatography and Tandem Mass Spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of Antioxidant Activity, Total Phenols and Phenolic Compounds in Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Marjoram (Origanum majorana L.) Extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant Properties and Composition of Aqueous Extracts from Mentha Species, Hybrids, Varieties, and Cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Pegg, R.B.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J.A. Free-Radical Scavenging Capacity and Antioxidant Activity of Selected Plant Species from the Canadian Prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main Bioactive Phenolic Compounds in Marine Algae and Their Mechanisms of Action Supporting Potential Health Benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Gaspar, A.; Martins, M.; Silva, P.; Garrido, E.M.; Garrido, J.; Firuzi, O.; Miri, R.; Saso, L.; Borges, F. Dietary Phenolic Acids and Derivatives. Evaluation of the Antioxidant Activity of Sinapic Acid and Its Alkyl Esters. J. Agric. Food Chem. 2010, 58, 11273–11280. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of Phenolic Compounds in Greek Plants of Lamiaceae Family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; Martínez-Huélamo, M.; Rinaldi Alvarenga, J.F.; Leal, L.N.; Lamuela-Raventos, R.M. A Comprehensive Study on the Phenolic Profile of Widely Used Culinary Herbs and Spices: Rosemary, Thyme, Oregano, Cinnamon, Cumin and Bay. Food Chem. 2014, 154, 299–307. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Bisha, B.; Weinsetel, N.; Brehm-Stecher, B.F.; Mendonca, A. Antilisterial Effects of Gravinol-S Grape Seed Extract at Low Levels in Aqueous Media and Its Potential Application as a Produce Wash. J. Food Prot. 2010, 73, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Cunniff, P.A. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1995; ISBN 0935584544. [Google Scholar]
- Ozdal, T.; Capanoglu, E.; Altay, F. A Review on Protein-Phenolic Interactions and Associated Changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al-Gharibi, I.; Al-Abri, A.; Al-Humaimi, A.; Al-Nabhani, F.; Al-Hashmi, H.; Al-Sarmi, K.; Al-Shibli, S. Evaluating the Shelf-Life of Pasteurized Milk in Oman. Heliyon 2021, 7, e06555. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.T.; Tahergorabi, R. Milk Bacteria and Gastrointestinal Tract: Microbial Composition of Milk; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128144695. [Google Scholar]
- Dogan, B.; Boor, K.J. Genetic Diversity and Spoilage Potentials among Pseudomonas Spp. Isolated from Fluid Milk Products and Dairy Processing Plants. Appl. Environ. Microbiol. 2003, 69, 130–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.Y.; Kim, H.J.; Lee, K.A.; Kim, K.T.; Paik, H.D. Antimicrobial Activity of Acid-Hydrolyzed Citrus Unshiu Peel Extract in Milk. J. Dairy Sci. 2014, 97, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.L.; Choi, N.H.; Bajpai, V.K.; Kang, S.C. Synergistic Effect of Nisin and Garlic Shoot Juice against Listeria monocytogenes in Milk. Food Chem. 2008, 110, 375–382. [Google Scholar] [CrossRef]
- Zamuz, S.; Munekata, P.E.S.; Dzuvor, C.K.O.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The Role of Phenolic Compounds against Listeria monocytogenes in Food. A Review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Ben Jemaa, M.; Falleh, H.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Quality Preservation of Deliberately Contaminated Milk Using Thyme Free and Nanoemulsified Essential Oils. Food Chem. 2017, 217, 726–734. [Google Scholar] [CrossRef] [Green Version]
- Klaric, M.; Oven, P.; Gorišek, Ž.; Španic, N.; Pervan, S. Yield of Stirred Cold Maceration and Extraction of Milled European Black Alder Wood and Bark Using Different Solvents. BioResources 2016, 11, 9244–9254. [Google Scholar] [CrossRef] [Green Version]
- Mokrani, A.; Madani, K. Effect of Solvent, Time and Temperature on the Extraction of Phenolic Compounds and Antioxidant Capacity of Peach (Prunus persica L.) Fruit; Elsevier B.V.: Amsterdam, The Netherlands, 2016; Volume 162, ISBN 2135415065. [Google Scholar]
- Olatunji, T.L.; Afolayan, A.J. Comparative Quantitative Study on Phytochemical Contents and Antioxidant Activities of Capsicum annuum L. and Capsicum frutescens L. Sci. World J. 2019, 2019, 4705140. [Google Scholar] [CrossRef] [Green Version]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of Antioxidant, Anti-Inflammatory, Anti-Cholinesterase and Cytotoxic Activities of Pomegranate (Punica granatum) Leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef]
- Oke-Altuntas, F.; Ipekcioglu, S.; Yaglioglu, A.S.; Behcet, L.; Demirtas, I. Phytochemical Analysis, Antiproliferative and Antioxidant Activities of Chrozophora Tinctoria: A Natural Dye Plant. Pharm. Biol. 2017, 55, 966–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical Composition, Biological and Cytotoxic Activities of Cistus Salviifolius Flower Buds and Leaves Extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- AlMahmud, Z.; Bachar, S.C.; Hasan, C.M.; Bin Emran, T.; Qais, N.; Uddin, M.M.N. Phytochemical Investigations and Antioxidant Potential of Roots of Leea macrophylla (Roxb.). BMC Res. Notes 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Sassi Aydi, S.; Aydi, S.; Ben Abdallah Kolsi, R.; Haddeji, N.; Rahmani, R.; Ktari, N.; Bouajila, J. CO2 Enrichment: Enhancing Antioxidant, Antibacterial and Anticancer Activities in Arthrospira Platensis. Food Biosci. 2020, 35, 100575. [Google Scholar] [CrossRef]
- Cerretani, L.; Bendini, A. Rapid Assays to Evaluate the Antioxidant Capacity of Phenols in Virgin Olive Oil; Elsevier Inc.: Amsterdam, The Netherlands, 2010; ISBN 9780123744203. [Google Scholar]
- Ben Jalloul, A.; Chaar, H.; Tounsi, M.S.; Abderrabba, M. Variations in Phenolic Composition and Antioxidant Activities of Scabiosa maritima (Scabiosa atropurpurea sub. maritima L.) Crude Extracts and Fractions According to Growth Stage and Plant Part. S. Afr. J. Bot. 2022, 146, 703–714. [Google Scholar] [CrossRef]




| Trait | |||||||
|---|---|---|---|---|---|---|---|
| Extracts | TPC | TFC | CTC | ABTSIC50 | DPPHIC50 | TAC | RP |
| Thyme | 104.23 a(±4.79) | 73.91 a(±4.34) | 10.98 a(±0.86) | 24.56 c(±2.30) | 30.44 c(±4.79) | 459.50 a(±27.57) | 519.02 a(±5.16) |
| Rosemary | 32.65 c(±0.46) | 14.56 b(±0.78) | 10.07 a(±0.05) | 104.55 a(±4.57) | 259.16 a(±4.79) | 155.42 b(±12.37) | 53.54 b(±2.93) |
| Ammoides | 74.49 b(±0.85) | 22.15 b(±5.60) | 3.25 b(±0.05) | 37.86 b(±4.79) | 122.41 b(±1.63) | 154.79 b(±4.60) | 30.12 c(±0.17) |
| Fortified milk serum DPPH (%) | |||||||
| Control | Thyme | Rosemary | Ammoides | ||||
| [Extract] = 0.1 mg/mL | 10.69 d(±1.67) | 74.84 a(±0.33) | 68.68 b(±0.11) | 45.91 c(±0.00) | |||
| [Extract] = 0.5 mg/mL | 10.69 b(±1.67) | 85 a(±1.08) | 82 a(±1.00) | 83 a(±2.00) | |||
| Thyme | Rosemary | Ammoides | |
|---|---|---|---|
| Simple phenols: | |||
| Resorcinol | 24.75 | 17.08 | ND |
| Catechol | 12.93 | 9.31 | ND |
| Phenolic acid: | |||
| Gallic acid | 1.59 | 2.01 | ND |
| Chlorogenic acid | 20.23 | 32.14 | 38.30 |
| Caffeic acid | 15.75 | 48.78 | 80.23 |
| Syringic acid | 7.35 | 5.03 | 8.83 |
| p-Coumaric acid | 10.03 | 24.42 | 41.08 |
| Sinapic acid | 135.14 | 37.97 | 64.13 |
| Ferulic acid | 177.71 | 97.53 | 7.40 |
| Rosmarinic acid | 49.76 | 74.64 | 116.53 |
| Ellagic acid | 357.99 | ND | ND |
| Trans Cinnamic acid | 1.12 | ND | ND |
| Flavonoids: | |||
| Catechin hydrate | 131.96 | 1222.94 | 32.73 |
| Epicatechine-3-O-gallate | 51.93 | 57.21 | 74.39 |
| Isorhamnetin-3-O-glucoside | 32.03 | 13.46 | 22.41 |
| Rutin | 138.64 | ND | ND |
| Isoquerci0in | ND | 19.81 | 26.40 |
| Kaempferol-3-O-rutinoside | ND | 8.72 | 15.24 |
| Isorhamnetin-3-O-rutinoside | 33.64 | ND | 5.87 |
| Quercetin | 13.71 | ND | ND |
| Luteolin | 4.29 | 1.42 | ND |
| Kaempferol | 13.94 | ND | ND |
| Stilbene: | |||
| Resveratrol | ND | 21.27 | 31.15 |
| Unknown | 1683.45 | 2747.82 | 1409.38 |
| Total | 2917.94 | 4441.72 | 1974.07 |
| Factor | Associative Effect of Extracts and Heat Treatments | Extracts Effect on Stored Pasteurized Milk | ||||||
|---|---|---|---|---|---|---|---|---|
| TVC (Log CFU/mL) | TPC (Log CFU/mL) | TVC (Log CFU/mL) | TPC (Log CFU/mL) | |||||
| F | p-Value | F | p-Value | F | p-Value | F | p-Value | |
| Test of between-subject effects | ||||||||
| Extract | 1458.558 | <0.001 *** | 647.267 | <0.001 *** | 150.682 | <0.001 *** | 324.574 | <0.001 *** |
| Dose | 10,007.332 | <0.001 *** | 277.660 | <0.001 *** | 3107.662 | <0.001 *** | 891.344 | <0.001 *** |
| Temperature | 865,519.019 | <0.001 *** | 18,535.986 | <0.001 *** | ||||
| Extract * Dose | 2145.419 | <0.001 *** | 36.844 | <0.001 *** | 29.530 | <0.001 *** | 34.607 | <0.001 *** |
| Temperature * Extract | 302.142 | <0.001 *** | 46.107 | <0.001 *** | ||||
| Temperature * Dose | 712.021 | <0.001 *** | 4.540 | 0.002 ** | ||||
| Temperature * Dose * Extract | 89.396 | <0.001 *** | 10.335 | <0.001 *** | ||||
| Test within subjects effects | ||||||||
| Time | 126.724 | <0.001 *** | 120.975 | <0.001 *** | 88.220 | <0.001 *** | 54.386 | <0.001 *** |
| Time * Extract | 7.474 | 0.002 ** | 24.130 | <0.001 *** | 1.596 | 0.219 | 3.286 | 0.035 * |
| Time * Dose | 9.139 | <0.001 *** | 3.606 | 0.022 * | 3.040 | 0.044 * | 11.947 | <0.001 *** |
| Time * Temperature | 16.345 | <0.001 *** | 3.728 | 0.034 * | ||||
| Time * Extract * Dose | 5.264 | 0.001 ** | 3.544 | 0.007 ** | 2.079 | 0.094 | 1.732 | 0.158 |
| Time * Temperature * Extract | 1.201 | 0.327 | 1.477 | 0.230 | ||||
| Time * Temperature * Dose | 3.293 | 0.011 * | 1.675 | 0.155 | ||||
| Time * Temperature * Dose * Extract | 2.849 | 0.007 ** | 1.493 | 0.172 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalloul, A.B.; Ayadi, N.; Klai, A.; Abderrabba, M. Functionalization of Pasteurized Milk Using Rosemary, Thyme, and Ammoides Aqueous Extracts for Better Microbial Quality and an Improved Antioxidant Activity. Molecules 2022, 27, 3725. https://doi.org/10.3390/molecules27123725
Jalloul AB, Ayadi N, Klai A, Abderrabba M. Functionalization of Pasteurized Milk Using Rosemary, Thyme, and Ammoides Aqueous Extracts for Better Microbial Quality and an Improved Antioxidant Activity. Molecules. 2022; 27(12):3725. https://doi.org/10.3390/molecules27123725
Chicago/Turabian StyleJalloul, Amel Ben, Nourhene Ayadi, Amira Klai, and Manef Abderrabba. 2022. "Functionalization of Pasteurized Milk Using Rosemary, Thyme, and Ammoides Aqueous Extracts for Better Microbial Quality and an Improved Antioxidant Activity" Molecules 27, no. 12: 3725. https://doi.org/10.3390/molecules27123725