Stability and Antiproliferative Activity of Malvidin-Based Non-Oxonium Derivative (Oxovitisin A) Compared with Precursor Anthocyanins and Pyranoanthocyanins
Abstract
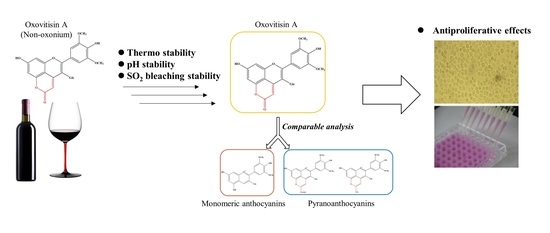
:1. Introduction
2. Results and Discussion
2.1. Stability of Oxovitisin A
2.2. Thermostability
2.3. pH Stability
2.4. Bisulfite Bleaching Stability
2.5. Antiproliferative Capacity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Vitisin A, Me-Py, and Oxovitisin A
3.3. Purification of Mv3glc and Its Derivatives
3.4. Thermal Stability
3.5. pH and Color Stability
3.6. SO2 Bleaching Stability
3.7. Cell Culture Conditions
3.8. Sulforhodamine B Assay
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bueno, J.M.; Saez-Plaza, P.; Ramos-Escudero, F.; Jimenez, A.M.; Fett, R.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part II: Chemical structure, color, and intake of anthocyanins. Crit. Rev. Anal. Chem. 2012, 42, 126–151. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboonabi, A.; Meyer, R.R.; Gaiz, A.; Singh, I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020, 76, 82–93. [Google Scholar] [CrossRef]
- Oliveira, H.; Fernandes, A.; Brás, N.F.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as antidiabetic agents-in vitro and in silico approaches of preventive and therapeutic effects. Molecules 2020, 25, 3813. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.C.; Murador, D.C.; Mesquita, L.M.D.; de Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compost. Anal. 2018, 68, 31–40. [Google Scholar] [CrossRef]
- Oliveira, J.; Fernandes, V.; Miranda, C.; Santos-Buelga, C.; Silva, A.; de Freitas, V.; Mateus, N. Color properties of four cyanidin-pyruvic acid adducts. J. Agric. Food. Chem. 2006, 54, 6894–6903. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins―An overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Schwarz, M.; Winterhalter, P. A novel synthetic route to substituted pyranoanthocyanins with unique colour properties. Tetrahedron Lett. 2003, 44, 7583–7587. [Google Scholar] [CrossRef]
- He, J.; Carvalho, A.R.; Mateus, N.; De Freitas, V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food. Chem. 2010, 58, 9249–9258. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Luo, H.; Ding, L.; Jiang, X.; Li, X.; Jiao, R.; Bai, W. Comparative study on the stability and antioxidant activity of six pyranoanthocyanins based Malvidin-3-glucoside. J. Agric. Food. Chem. 2020, 68, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Oliveira, J.; Silva, A.M.; Mateus, N.; De Freitas, V. Oxovitisins: A new class of neutral pyranone-anthocyanin derivatives in red wines. J. Agric. Food. Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef]
- He, J.; Silva, A.M.; Mateus, N.; de Freitas, V. Oxidative formation and structural characterisation of new alpha-pyranone (lactone) compounds of non-oxonium nature originated from fruit anthocyanins. Food Chem. 2011, 127, 984–992. [Google Scholar] [CrossRef]
- Alcaro, S.; Chiodo, S.G.; Leopoldini, M.; Ortuso, F. Antioxidant efficiency of oxovitisin, a new class of red wine pyranoanthocyanins, revealed through quantum mechanical investigations. J. Chem. Inf. Model. 2013, 53, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, J.; Oliveira, J.; Cruz, L.; Teixeira, N.; Bras, N.F.; De Freitas, V.; Mateus, N. Antioxidant features of red wine pyranoanthocyanins: Experimental and theoretical approaches. J. Agric. Food. Chem. 2014, 62, 7002–7009. [Google Scholar] [CrossRef]
- Im, N.K.; Jang, W.J.; Jeong, C.H.; Jeong, G.S. Delphinidin suppresses PMA-induced MMP-9 expression by blocking the NF-kappaB activation through MAPK signaling pathways in MCF-7 human breast carcinoma cells. J. Med. Food 2014, 17, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.X.; Kai, K.; Li, J.J.; Lin, S.; Terahara, N.; Wakamatsu, M.; Fujii, M.; Young, M.R.; Colburn, N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: Structure-activity relationship and molecular mechanisms. Carcinogenesis 2004, 25, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.; Fernandes, I.; de Freitas, V.; Mateus, N. Ageing impact on the antioxidant and antiproliferative properties of Port wines. Food Res. Int. 2015, 67, 199–205. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, F.; de Freitas, V.; Mateus, N. Antioxidant and antiproliferative properties of methylated metabolites of anthocyanins. Food Chem. 2013, 141, 2923–2933. [Google Scholar] [CrossRef]
- Fernandes, I.; Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Bioavailability of anthocyanins and derivatives. J. Funct. Foods 2014, 7, 54–66. [Google Scholar] [CrossRef]
- Oliveira, H.; Wu, N.; Zhang, Q.; Wang, J.; Oliveira, J.; de Freitas, V.; Mateus, N.; He, J.; Fernandes, I. Bioavailability studies and anticancer properties of malvidin based anthocyanins, pyranoanthocyanins and non-oxonium derivatives. Food Funct. 2016, 7, 2462–2468. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro absorption and antiproliferative activities of monomeric and polymeric anthocyanin fractions from açai fruit (Euterpe oleracea Mart.). Food Chem. 2010, 119, 1071–1078. [Google Scholar] [CrossRef]
- Anwar, S.; Fratantonio, D.; Ferrari, D.; Saija, A.; Cimino, F.; Speciale, A. Berry anthocyanins reduce proliferation of human colorectal carcinoma cells by inducing caspase-3 activation and p21 upregulation. Mol. Med. Report. 2016, 14, 1397–1403. [Google Scholar] [CrossRef] [Green Version]
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the cancer cell growth inhibitory effects of table grape anthocyanins. J. Food Sci. 2018, 83, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Navindra, P.S.; Leslie, D.B.; Muraleedharan, G.N. Degradation products of cyanidin glycosides from tart cherries and their bioactivities. J. Agric. Food. Chem. 2001, 49, 4924–4929. [Google Scholar]
- Sousa, A.; Araujo, P.; Azevedo, J.; Cruz, L.; Fernandes, I.; Mateus, N.; de Freitas, V. Antioxidant and antiproliferative properties of 3-deoxyanthocyanidins. Food Chem. 2016, 192, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Guohua, C.; Ronald, L.P. Oxygen radical absorbing capacity of anthocyanins. J. Agric. Food. Chem. 1997, 45, 304–309. [Google Scholar]
- Faria, A.; Pestana, D.; Azevedo, J.; Martel, F.; de Freitas, V.; Azevedo, I.; Mateus, N.; Calhau, C. Absorption of anthocyanins through intestinal epithelial cells―Putative involvement of GLUT2. Mol. Nutr. Food Res. 2009, 53, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Faria, A.; de Freitas, V.; Calhau, C.; Mateus, N. Multiple-approach studies to assess anthocyanin bioavailability. Phytochem. Rev. 2015, 14, 899–919. [Google Scholar] [CrossRef]
- Oliveira, H.; Roma-Rodrigues, C.; Santos, A.; Veigas, B.; Bras, N.; Faria, A.; Calhau, C.; de Freitas, V.; Baptista, P.V.; Mateus, N.; et al. GLUT1 and GLUT3 involvement in anthocyanin gastric transport-Nanobased targeted approach. Sci. Rep. 2019, 9, 789. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Perez-Gregorio, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in vitro gastrointestinal bioavailability of acylated and non-acylated anthocyanins: Purple-fleshed sweet potato vs red wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef]
- Oliveira, H.; Fernandes, I.; Bras, N.F.; Faria, A.; De Freitas, V.; Calhau, C.; Mateus, N. Experimental and theoretical data on the mechanism by which red wine anthocyanins are transported through a human MKN-28 gastric cell model. J. Agric. Food. Chem. 2015, 63, 7685–7692. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wu, N.; Kuang, M.; Lamikanra, O.; Liu, G.; Li, S.; He, J. Preparation and toxicological evaluation of methyl pyranoanthocyanin. Food Chem. Toxicol. 2015, 83, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Wang, J.; Jiang, T.; Li, S.; Zhu, Z.; He, J. Preparation, spectral properties and antioxidant activities of pyranone-anthocyanin derivative (Oxovitisin). Spectrosc Spect Anal. 2017, 37, 2120–2127. [Google Scholar]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Thermal stability of anthocyanins and colourless phenolics in pomegranate (Punica granatum L.) juices and model solutions. Food Chem. 2013, 138, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.D.; Zhang, Y.; Zheng, Z.D.; Qu, Z.Y.; Liu, M.; Zhu, S.H.; Liu, S.; Wang, M.; Qu, L. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef]





| Mv3glc | |||||||||
| L* | a* | b* | C* | ha,b | ΔL* | ΔC* | Δha,b | ΔE* | |
| pH 1.0 | 29.12 | 7.54 | −0.05 | 7.54 | 359.61 | - | - | - | - |
| pH 2.5 | 30.21 | 5.45 | −0.43 | 5.47 | 355.46 | 1.08 | −2.07 | −4.15 | 2.39 |
| pH 3.6 | 31.22 | 2.30 | −0.55 | 2.36 | 346.62 | 2.09 | −5.18 | −12.99 | 5.67 |
| pH 4.5 | 31.19 | 1.39 | −0.73 | 1.57 | 332.3 | 2.07 | −5.97 | −27.31 | 6.53 |
| pH 5.5 | 31.28 | 1.21 | −0.76 | 1.43 | 327.95 | 2.16 | −6.11 | −31.65 | 6.73 |
| pH 7.0 | 31.35 | 1.44 | −0.69 | 1.60 | 334.39 | 2.22 | −5.94 | −25.21 | 6.53 |
| pH 9.0 | 31.37 | 0.51 | −0.52 | 0.73 | 314.70 | 2.25 | −6.81 | −44.91 | 7.40 |
| pH 11.0 | 29.03 | −1.64 | 1.11 | 1.98 | 145.91 | −0.1 | −5.56 | −213.7 | 9.26 |
| Vitisin A | |||||||||
| L* | a* | b* | C* | ha,b | ΔL* | ΔC* | Δha,b | ΔE* | |
| pH 1.0 | 23.74 | 7.52 | −2.58 | 7.95 | 341.06 | - | - | - | - |
| pH 2.5 | 24.76 | 8.96 | 0.7 | 8.99 | 4.47 | 1.02 | 1.04 | −336.59 | 3.72 |
| pH 3.6 | 25.52 | 8.84 | 2.85 | 9.28 | 17.87 | 1.78 | 1.33 | −323.19 | 5.86 |
| pH 4.5 | 25.71 | 9.16 | 3.1 | 9.67 | 18.71 | 1.97 | 1.72 | −322.35 | 6.23 |
| pH 5.5 | 25.25 | 7.63 | 1.92 | 7.87 | 14.13 | 1.51 | −0.08 | −0.08 | 4.75 |
| pH 7.0 | 24.11 | 3.49 | 0.03 | 3.49 | 0.54 | 0.37 | −4.46 | −4.46 | 4.82 |
| pH 9.0 | 23.65 | 1.31 | −0.68 | 1.48 | 332.57 | −0.09 | −6.47 | −6.47 | 6.49 |
| pH 11.0 | 23.45 | −2.92 | −1.51 | 3.29 | 207.37 | −0.29 | −4.66 | −4.66 | 10.50 |
| Me-py | |||||||||
| L* | a* | b* | C* | ha,b | ΔL* | ΔC* | Δha,b | ΔE* | |
| pH 1.0 | 31.85 | 0.89 | 11.28 | 11.31 | 85.51 | - | - | - | - |
| pH 2.5 | 31.83 | 0.76 | 11.32 | 11.35 | 86.15 | −0.02 | 0.04 | 0.64 | 0.13 |
| pH 3.6 | 31.8 | 0.89 | 11.2 | 11.23 | 85.45 | −0.05 | −0.08 | −0.06 | 0.1 |
| pH 4.5 | 31.41 | 1.9 | 10.21 | 10.38 | 79.47 | −0.44 | −0.93 | −6.04 | 1.54 |
| pH 5.5 | 31.17 | 2.52 | 9.43 | 9.76 | 75.01 | −0.68 | −1.55 | −10.5 | 2.57 |
| pH 7.0 | 30.76 | 3.16 | 8.24 | 8.83 | 69.05 | −1.09 | −2.84 | −16.46 | 3.94 |
| pH 9.0 | 27.16 | 4.35 | 1.10 | 4.48 | 14.27 | −4.69 | −6.83 | −71.24 | 11.72 |
| pH 11.0 | 25.18 | 4.53 | −4.64 | 6.49 | 314.33 | −6.67 | −4.82 | 228.82 | 17.64 |
| Oxovitisin A | |||||||||
| L* | a* | b* | C* | ha,b | ΔL* | ΔC* | Δha,b | ΔE* | |
| pH 1.0 | 33.99 | −1.31 | 2.07 | 2.45 | 122.38 | - | - | - | - |
| pH 2.5 | 34.01 | −1.42 | 2.27 | 2.68 | 122.07 | 0.02 | 0.23 | −0.31 | 0.23 |
| pH 3.6 | 34.06 | −1.47 | 2.27 | 2.7 | 123.01 | 0.07 | 0.25 | 0.63 | 0.27 |
| pH 4.5 | 34.1 | −1.51 | 2.26 | 2.71 | 123.73 | 0.11 | 0.26 | 1.35 | 0.29 |
| pH 5.5 | 34.04 | −1.89 | 3.54 | 4.02 | 118.12 | 0.05 | 1.57 | −4.26 | 1.58 |
| pH 7.0 | 33.93 | −1.85 | 3.32 | 3.8 | 119.13 | −0.06 | 1.35 | −3.25 | 1.37 |
| pH 9.0 | 33.33 | −2.78 | 9.82 | 10.2 | 105.8 | −0.66 | 7.75 | −16.58 | 7.92 |
| pH 11.0 | 32.71 | −1.74 | 12.81 | 12.93 | 97.74 | −1.28 | 10.48 | −24.64 | 10.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Ma, Y.; Li, A.; Wang, J.; He, J.; Zhang, R. Stability and Antiproliferative Activity of Malvidin-Based Non-Oxonium Derivative (Oxovitisin A) Compared with Precursor Anthocyanins and Pyranoanthocyanins. Molecules 2022, 27, 5030. https://doi.org/10.3390/molecules27155030
Wu M, Ma Y, Li A, Wang J, He J, Zhang R. Stability and Antiproliferative Activity of Malvidin-Based Non-Oxonium Derivative (Oxovitisin A) Compared with Precursor Anthocyanins and Pyranoanthocyanins. Molecules. 2022; 27(15):5030. https://doi.org/10.3390/molecules27155030
Chicago/Turabian StyleWu, Muci, Yan Ma, Ao Li, **gyi Wang, **gren He, and Rui Zhang. 2022. "Stability and Antiproliferative Activity of Malvidin-Based Non-Oxonium Derivative (Oxovitisin A) Compared with Precursor Anthocyanins and Pyranoanthocyanins" Molecules 27, no. 15: 5030. https://doi.org/10.3390/molecules27155030