Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor
Abstract
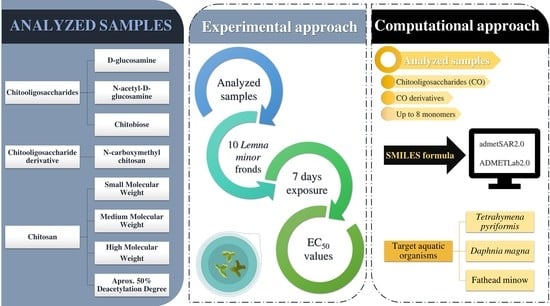
:1. Introduction
2. Results and Discussions
2.1. Effects of Chitosan, Chitooligosaccharides and Carboxymethyl Chitosan on Lemna minor
2.2. Predictions of the Effects of COs and Their Derivatives on Aquatic Organisms
3. Materials and Methods
3.1. Materials Used in the Experimental Approach
3.2. Materials Considered in the Computational Approach
3.3. Lemna minor Growth Inhibition Assay
3.4. Computational Assessment of the Effects of COs on the Aquatic Organisms
3.5. Statistical Analysis Used in the Experimental Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhao, L.-M.; Shi, L.-E.; Zhang, Z.-L.; Chen, J.-M.; Shi, D.-D.; Yang, J.; Tang, Z.-X. Preparation and application of chitosan nanoparticles and nanofibers. Braz. J. Chem. Eng. 2011, 28, 353–362. [Google Scholar] [CrossRef]
- Kou, S.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohyd. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan biomaterials application in dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zúñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and its potential use as a scaffold for tissue engineering in regenerative medicine. BioMed Res. Int. 2015, 2015, 821279. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Boros, B.-V.; Ostafe, V. Evaluation of ecotoxicology assessment methods of nanomaterials and their effects. Nanomaterials 2020, 10, 610. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Tan, T.; Yuan, H.; Rui, C. Insecticidal and Fungicidal Activities of Chitosan and Oligo-chitosan. J. Bioact. Compat. Polym. 2003, 18, 391–400. [Google Scholar] [CrossRef]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New chitin, chitosan, and O-carboxymethyl chitosan sources from resting eggs of Daphnia longispina (Crustacea); with physicochemical characterization, and antimicrobial and antioxidant activities. Biotechnol. Bioproc. E 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Yuan, Y.; Xu, D.; Kang, X. Enantioselective ecotoxicity of the herbicide dichlorprop and complexes formed with chitosan in two fresh water green algae. J. Environ. Monitor. 2011, 13, 879–885. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; **, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Nam, J.-P.; Nah, J.-W. Application of chitosan and chitosan derivatives as biomaterials. J. Ind. Eng. Chem. 2016, 33, 1–10. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mater. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A.; Muzzarelli, C.; Tarsi, R.; Miliani, M.; Gabbanelli, F.; Cartolari, M. Fungistatic activity of modified chitosans against Saprolegnia parasitica. Biomacromolecules 2001, 2, 165–169. [Google Scholar] [CrossRef]
- Xu, Q.; Dou, J.; Wei, P.; Tan, C.; Yun, X.; Wu, Y.; Bai, X.; Ma, X.; Du, Y. Chitooligosaccharides induce apoptosis of human hepatocellular carcinoma cells via up-regulation of Bax. Carbohyd. Polym. 2008, 71, 509–514. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and their biological activities: A comprehensive review. Carbohyd. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef]
- Hamer, S.N.; Cord-Landwehr, S.; Biarnés, X.; Planas, A.; Waegeman, H.; Moerschbacher, B.M.; Kolkenbrock, S. Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Sci. Rep. 2015, 5, 8716. [Google Scholar] [CrossRef]
- Il’ina, A.V.; Varlamov, V.P. In vitro antitumor activity of heterochitooligosaccharides (Review). Appl. Biochem. Micro+ 2015, 51, 1–10. [Google Scholar] [CrossRef]
- Crosino, A.; Moscato, E.; Blangetti, M.; Carotenuto, G.; Spina, F.; Bordignon, S.; Puech-Pagès, V.; Anfossi, L.; Volpe, V.; Prandi, C.; et al. Extraction of short chain chitooligosaccharides from fungal biomass and their use as promoters of arbuscular mycorrhizal symbiosis. Sci. Rep. 2021, 11, 3798. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968–987. [Google Scholar] [CrossRef] [PubMed]
- Faqir, Y.; Ma, J.; Chai, Y. Chitosan in modern agriculture production. Plant Soil Environ. 2021, 67, 679–699. [Google Scholar] [CrossRef]
- Roman, D.L.; Roman, M.; Som, C.; Schmutz, M.; Hernandez, E.; Wick, P.; Casalini, T.; Perale, G.; Ostafe, V.; Isvoran, A. Computational assessment of the pharmacological profiles of degradation products of chitosan. Front Bioeng. Biotechnol. 2019, 214. [Google Scholar] [CrossRef]
- Isvoran, A.; Aurel Ciorsac, A.; Ostafe, V. ADME-Tox profiling of some low molecular weight water soluble chitosan derivatives. ADMET DMPK 2017, 5, 192–200. [Google Scholar] [CrossRef] [Green Version]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Computational Assessment of Chito-Oligosaccharides Interactions with Plasma Proteins. Mar. Drugs 2021, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Roman, M.; Sletta, H.; Ostafe, V.; Isvoran, A. Assessment of the properties of chitin deacetylases showing different enzymatic action patterns. J. Mol. Graph. Model. 2019, 88, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Ostafe, V.; Isvoran, A. Deeper inside the specificity of lysozyme when degrading chitosan. A structural bioinformatics study. J. Mol. Graph. Model. 2020, 100, 107676. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development. Test No. 221: Lemna sp. Growth Inhibition Test. In OECD Guidelines for the Testing of Chemicals; 2006. [Google Scholar]
- Boros, B.-V.; Grau, N.I.; Isvoran, A.; Datcu, A.D.; Ianovici, N.; Ostafe, V. A study of the effects of sodium alginate and sodium carboxymethyl cellulose on the growth of common duckweed (Lemna minor L.): Scientific paper. J. Serb. Chem. Soc. 2022, 87, 657–667. [Google Scholar] [CrossRef]
- Luechtefeld, T.; Hartung, T. Computational approaches to chemical hazard assessment. ALTEX-Altern Anim. Ex. 2017, 34, 459–478. [Google Scholar] [CrossRef] [PubMed]
- Lo Piparo, E.; Worth, A. Review of QSAR Models and Software Tools for Predicting Developmental and Reproductive Toxicity; JRC Report EUR 24522 EN; Publications Office of the European Union: Luxembourg, 2010.
- Dascălu, D.; Roman, D.L.; Filip, M.; Ciorsac, A.A.; Ostafe, V.; Isvoran, A. Solubility and ADMET profiles of short oligomers of lactic acid. ADMET DMPK 2020, 8, 425–436. [Google Scholar] [CrossRef]
- Roman, D.L.; Isvoran, A.; Filip, M.; Ostafe, V.; Zinn, M. In silico Assessment of Pharmacological Profile of Low Molecular Weight Oligo-Hydroxyalkanoates. Front. Bioeng. Biotechnol. 2020, 8, 584010. [Google Scholar] [CrossRef]
- Roman, M.; Roman, D.L.; Ostafe, V.; Ciorsac, A.; Isvoran, A. Computational Assessment of Pharmacokinetics and Biological Effects of Some Anabolic and Androgen Steroids. Pharm. Res. 2018, 35, 41. [Google Scholar] [CrossRef]
- Voiculescu, D.I.; Ostafe, V.; Isvoran, A. Computational assessment of the pharmacokinetics and toxicity of the intensive sweeteners. Farmacia 2021, 69, 1032–1041. [Google Scholar] [CrossRef]
- Craciun, D.; Modra, D.; Isvoran, A. ADME-Tox profiles of some food additives and pesticides. In AIP Conference Proceedings; AIP Publishing LLC: Baltimore, MD, USA, 2015; Volume 1694, p. 040007. [Google Scholar]
- Gridan, I.M.; Ciorsac, A.A.; Isvoran, A. Prediction of ADME-Tox properties and toxicological endpoints of triazole fungicides used for cereals protection. ADMET DMPK 2019, 7, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Voiculescu, D.I.; Roman, D.L.; Ostafe, V.; Isvoran, A. A Cheminformatics Study Regarding the Human Health Risks Assessment of the Stereoisomers of Difenoconazole. Molecules 2022, 27, 4682. [Google Scholar] [CrossRef] [PubMed]
- Roman, D.L.; Voiculescu, D.I.; Matica, M.A.; Baerle, V.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Assessment of the effects of triticonazole on the soil and on the human health. Molecules 2022. [Google Scholar]
- Alves, V.M.; Muratov, E.N.; Zakharov, A.; Muratov, N.N.; Andrade, C.H.; Tropsha, A. Chemical toxicity prediction for major classes of industrial chemicals: Is it possible to develop universal models covering cosmetics, drugs, and pesticides? Food Chem. Toxicol. 2018, 112, 526–534. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Technical Overview of Ecological Risk Assessment—Analysis Phase: Ecological Effects Characterization. Available online: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/technical-overview-ecological-risk-assessment-0 (accessed on 19 June 2022).
- Latowski, D.; Banaś, A.K.; Strzałka, K.; Gabryś, H. Amino sugars—New inhibitors of zeaxanthin epoxidase, a violaxanthin cycle enzyme. J. Plant Physiol. 2007, 164, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.; Ullrich, C.I. Uptake of Acidic and Basic Sugar Derivatives in Lemna gibba G1 1. Plant Physiol. 1989, 90, 1532–1537. [Google Scholar] [CrossRef]
- Cheng, F.; Li, W.; Zhou, Y.; Shen, J.; Wu, Z.; Liu, G.; Lee, P.W.; Tang, Y. admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. J. Chem. Inf. Model. 2012, 52, 3099–3105. [Google Scholar] [CrossRef]
- Yang, H.; Lou, C.; Sun, L.; Li, J.; Cai, Y.; Wang, Z.; Li, W.; Liu, G.; Tang, Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics 2019, 35, 1067–1069. [Google Scholar] [CrossRef]
- Bichsel, Y.; von Gunten, U. Formation of Iodo-Trihalomethanes during Disinfection and Oxidation of Iodide-Containing Waters. Environ. Sci. Technol. 2000, 34, 2784–2791. [Google Scholar] [CrossRef]
- Cheng, F.; Shen, J.; Yu, Y.; Li, W.; Liu, G.; Lee, P.W.; Tang, Y. In silico prediction of Tetrahymena pyriformis toxicity for diverse industrial chemicals with substructure pattern recognition and machine learning methods. Chemosphere 2011, 82, 1636–1643. [Google Scholar] [CrossRef]
- Su, L.; Fu, L.; He, J.; Qin, W.; Sheng, L.; Abraham, M.H.; Zhao, Y.H. Comparison of Tetrahymena pyriformis toxicity based on hydrophobicity, polarity, ionization and reactivity of class-based compounds. SAR QSAR Environ. Res. 2012, 23, 537–552. [Google Scholar] [CrossRef]
- Dong, J.; Wang, N.-N.; Yao, Z.-J.; Zhang, L.; Cheng, Y.; Ouyang, D.; Lu, A.-P.; Cao, D.-S. ADMETlab: A platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminformatics 2018, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- **ong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Malik, M.Y.H.; Binyamin, M.A.; Hayat, S. Correlation ability of degree-based topological indices for physicochemical properties of polycyclic aromatic hydrocarbons with applications. Polycycl. Aromat. Compd. 2022, in press. [Google Scholar] [CrossRef]
- Adnan, M.; Bokhary, S.A.U.H.; Abbas, G.; Iqbal, T. Degree-based topological indices and QSPR analysis of antituberculosis drugs. J. Chem. 2022, 5748626. [Google Scholar] [CrossRef]
- Wiener, H. Structural determination of paraffin boiling points. J. Am. Chem. Soc. 1947, 69, 17–20. [Google Scholar] [CrossRef]
- Gutman, I.; Krtvlyesi, T. Wiener indices and molecular surfaces. Zeitschrift Naturforschung A 1995, 50, 6696671. [Google Scholar] [CrossRef]
- Bajaj, S.; Sambi, S.S.; Madan, A.K. Predicting anti-HIV activity of phenethylthiazolethiourea (PETT) analogs: Computational approach using Wiener’s topochemical index. J. Mol. Struct. 2004, 684, 197–203. [Google Scholar] [CrossRef]
- Dureja, H.; Madan, A.K. Predicting Anti-HIV Activity of Dimethylaminopyridin-2-ones: Computational Approach using Topochemical Descriptors. Chem. Biol. Drug Des. 2009, 73, 258–270. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2020, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- AAT Bioquest Inc. Quest Graph™ EC50 Calculator. Available online: https://www.aatbio.com/tools/ec50-calculator (accessed on 25 July 2022).






| COs/COs Derivatives | MW (g/mol) | LogP | Probability to Produce | pIGC50 (µg/L) | |
|---|---|---|---|---|---|
| Crustacea Toxicity | Fish Aquatic Toxicity | ||||
| A | 179.170 | −2.143 | −0.790 | −0.959 | −1.446 |
| 2A | 340.330 | −3.032 | −0.710 | −0.875 | −1.066 |
| 3A | 501.480 | −3.658 | −0.710 | −0.865 | −0.959 |
| 4A | 662.640 | −4.545 | −0.710 | −0.865 | −0.766 |
| 5A | 823.790 | −5.178 | −0.710 | −0.865 | −0.738 |
| 6A | 984.950 | −6.099 | −0.710 | −0.865 | −0.730 |
| 8A | 1037.260 | −7.713 | −0.710 | −0.865 | −0.652 |
| D-glucosamine hydrochloride | 170.080 | −0.073 | −0.900 | −0.981 | −1.341 |
| chitobiose dihydrochloride | 340.150 | 0.212 | −0.880 | −0.977 | −0.963 |
| O-CMChi | 273.210 | −3.150 | −0.760 | −0.971 | −1.226 |
| N-CMChi | 295.240 | −3.470 | −0.740 | −0.959 | −0.778 |
| NO-CMChi | 353.280 | −3.630 | −0.705 | −0.922 | −0.806 |
| DaD | COs | MW (g/mol) | LogP | Cation Form | Neutral Form | ||||
|---|---|---|---|---|---|---|---|---|---|
| Probability to Produce | pIGC50 (µg/L) | Probability to Produce | pIGC50 (µg/L) | ||||||
| Crustacea Toxicity | Fish Aquatic Toxicity | Crustacea Toxicity | Fish Aquatic Toxicity | ||||||
| 33% | ADA | 585.560 | −3.332 | −0.690 | −0.917 | −1.000 | −0.700 | 0.871 | −0.925 |
| 50% | DA | 382.360 | −2.807 | −0.690 | −0.954 | −1.239 | −0.690 | −0.929 | −1.253 |
| DADA | 746.710 | −4.119 | −0.690 | −0.917 | −0.959 | −0.680 | −0.871 | −0.922 | |
| AADD | 746.710 | −4.119 | −0.690 | −0.917 | −0.921 | −0.700 | −0.871 | −0.862 | |
| DAAD | 746.710 | −4.073 | −0.690 | −0.917 | −0.798 | −0.680 | −0.871 | −0.838 | |
| ADDA | 746.710 | −4.194 | −0.690 | −0.917 | −0.871 | −0.700 | −0.871 | −0.846 | |
| ADADAD | 746.710 | −4.138 | −0.690 | −0.909 | −0.700 | −0.700 | −0.861 | −0.684 | |
| DADADA | 746.710 | −4.119 | −0.690 | −0.909 | −0.906 | −0.680 | −0.861 | −0.890 | |
| DADADADA | 1857.770 | −6.858 | −0.690 | −0.909 | −0.894 | −0.680 | −0.861 | −0.862 | |
| 67% | DDA | 523.520 | −3.664 | −0.690 | −0.954 | −1.015 | −0.680 | −0.929 | −0.969 |
| ADDDAD | 1069.020 | −5.772 | −0.690 | −0.917 | −0.650 | −0.700 | −0.871 | −0.664 | |
| DDDADA | 1069.020 | −5.737 | −0.690 | −0.917 | −0.904 | −0.680 | −0.871 | −0.895 | |
| 100% | D | 179.170 | −2.143 | −0.860 | −0.979 | −1.481 | −0.870 | −0.966 | −1.283 |
| 2D | 340.330 | −3.032 | −0.790 | −0.963 | −1.210 | −0.770 | −0.941 | −1.058 | |
| 3D | 501.480 | −3.658 | −0.790 | −0.963 | −0.885 | −0.770 | −0.941 | −0.909 | |
| 4D | 662.640 | −4.545 | −0.790 | −0.963 | −0.884 | −0.770 | −0.941 | −0.866 | |
| 5D | 823.790 | −5.178 | −0.790 | −0.963 | −0.821 | −0.770 | −0.9416 | −0.829 | |
| 6D | 984.950 | −6.099 | −0.790 | −0.963 | −0.848 | −0.770 | −0.9416 | −0.830 | |
| 8D | 1037.260 | −7.713 | −0.790 | −0.963 | −0.810 | −0.770 | −0.9416 | −0.807 | |
| Physicochemical Property/Deacetylation Degree | DaD = 0% | DaD = 100% | ||
|---|---|---|---|---|
| Equation | R2 | Equation | R2 | |
| MW | IGC50 = −0.0002 MW + 1.099 | 0.985 | IGC50 = −0.0007 MW + 1.420 | 0.978 |
| LC50FM = −0.0006 MW + 1.473 | 0.967 | LC50FM = −0.0004 MW + 1.602 | 0.936 | |
| LC50DM = −0.0048 MW +2.188 | 0.997 | LC50DM = −0.0002 MW + 2.335 | 0.242 | |
| logS | IGC50 = −1.040 logS + 0.350 | 0.978 | IGC50 = 0.348 logS + 1.700 | 0.833 |
| LC50FM = −0.707 logS + 1.083 | 0.909 | LC50FM = −0.186 logS + 1.462 | 0.888 | |
| LC50DM = −5.580 log S − 0.850 | 0.956 | LC50DM = −0.073 logS + 2.245 | 0.229 | |
| logP | IGC50 = 0.43 logP + 1.640 | 0.994 | IGC50 = −0.181 logP + 1.347 | 0.887 |
| LC50FM = 0.22 logP + 1.741 | 0.956 | LC50FM = 0.030 logP + 2.362 | 0.991 | |
| LC50DM = 1.750 logP + 4.340 | 0.998 | LC50DM = 0.067 logP + 1.664 | 0.998 | |
| Homo-Chitooligosaccharides | Hetero-Chitooligomers | Derivatives | |||
|---|---|---|---|---|---|
| AP = 0 | AP = 100% | AP = 33% | AP = 50% | AP = 67% | |
| 2D, 3D, 4D, 5D, 6D, 8D | 2A, 3A, 4A, 5A, 6A, 8A | DDA, DDDADA ADDDAD | DA, AADD, ADAD, ADDA, DAAD, DDAA, DADA, ADADAD, DADADA, DADADADA | ADA | G, 2G, O-CMChi, N-CMChi, NO-CMChi |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boros, B.-V.; Dascalu, D.; Ostafe, V.; Isvoran, A. Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor. Molecules 2022, 27, 6123. https://doi.org/10.3390/molecules27186123
Boros B-V, Dascalu D, Ostafe V, Isvoran A. Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor. Molecules. 2022; 27(18):6123. https://doi.org/10.3390/molecules27186123
Chicago/Turabian StyleBoros, Bianca-Vanesa, Daniela Dascalu, Vasile Ostafe, and Adriana Isvoran. 2022. "Assessment of the Effects of Chitosan, Chitooligosaccharides and Their Derivatives on Lemna minor" Molecules 27, no. 18: 6123. https://doi.org/10.3390/molecules27186123