Application of Cotton Swab–Ag Composite as Flexible Surface-Enhanced Raman Scattering Substrate for DMMP Detection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of ATS/AgNPs/CS
2.2. ATS/AgNPs/CS Conjugate Bonding Study
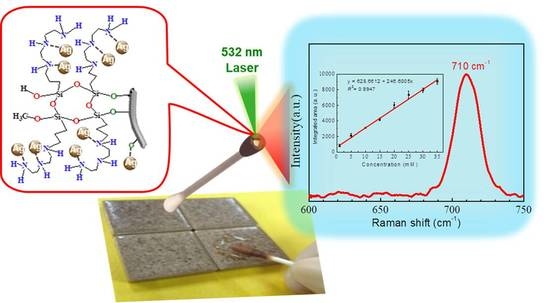
2.3. Sensitivity of ATS/AgNPs/CS
2.4. Reproducibility of ATS/AgNPs/CS
2.5. Stability of ATS/AgNPs/CS
3. Experimental
3.1. Materials and Apparatus
3.2. Synthesis of ATS-Stabilized AgNPs
3.3. Fabrication of SERS Cotton Swabs
3.4. Detection of DMMP Using ATS/AgNPs/CS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortiz-Rivera, W.; Pacheco-Londoño, L.C.; Hernández-Rivera, S.P. Remote Continuous Wave and Pulsed Laser Raman Detection of Chemical Warfare Agents Simulants and Toxic Industrial Compounds. Sens. Imaging 2010, 11, 131–145. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, Y.; Gao, J.; Chen, J.; Feng, J.; Guo, L.; **e, J. A simple and sensitive surface-enhanced Raman spectroscopic discriminative detection of organophosphorous nerve agents. Anal. Bioanal. Chem. 2017, 409, 5091–5099. [Google Scholar] [CrossRef]
- Baygildiev, T.M.; Vokuev, M.F.; Oreshkin, D.V.; Braun, A.V.; Godovikov, I.A.; Rybalchenko, I.V.; Rodin, I.A. p-Methoxyphenacyl Bromide as a Versatile Reagent for the Determination of Alkylphosphonic and Alkylmethylphosphonic Acids by High-Performance Liquid and Gas Chromatography with Mass Spectrometric Detection. J. Anal. Chem. 2020, 75, 1708–1718. [Google Scholar] [CrossRef]
- B’Hymer, C. A Brief Overview of HPLC–MS Analysis of Alkyl Methylphosphonic Acid Degradation Products of Nerve Agent. J. Chromatogr. Sci. 2019, 57, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Blanca, M.; Shifrovitch, A.; Madmon, M.; Elgarisi, M.; Dachir, S.; Lazar, S.; Baranes, S.; Egoz, I.; Avraham, M.; Jaoui, H.D.; et al. Retrospective determination of regenerated nerve agent sarin in human blood by liquid chromatography–mass spectrometry and in vivo implementation in rabbit. Arch. Toxicol. 2019, 13, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Chen, J.; Liu, H.; Lu, X.; Wu, J.; Zhang, Y.; Lin, Y.; Liu, Q.; Wang, H.; et al. Sensitive Untargeted Screening of Nerve Agents and Their Degradation Products Using Liquid Chromatography-High Resolution Mass Spectrometry. Anal. Chem. 2020, 92, 10578–10587. [Google Scholar] [CrossRef]
- Gäb, J.; Melzer, M.; Kehe, K.; Richardt, A.; Blum, M.M. Quantification of hydrolysis of toxic organophosphates and organophosphonates by diisopropyl fluorophosphatase from Loligo vulgaris by in situ Fourier transform infrared spectroscopy. Anal. Biochem. 2008, 385, 187–193. [Google Scholar] [CrossRef]
- Garg, P.; Pardasani, D.; Mazumder, A.; Purohit, A.; Dubey, D.K. Dispersive solid-phase extraction for in-sorbent Fourier-transform infrared detection and identification of nerve agent simulants in analysis for verification of chemical weapon convention. Anal. Bioanal. Chem. 2011, 399, 955–963. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, W.; Lin, Z.; Dong, J.; Zhen, N.; Chi, Y.; Hu, C. Mono- and Di-Sc-Substituted Keggin Polyoxometalates Effective Lewis Acid Catalysts for Nerve Agent Simulant Hydrolysis and Mechanistic Insights. Inorg. Chem. 2020, 59, 9756–9764. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Huang, F.; Dai, X.J.H.; Wu, W. Surface enhanced Raman scattering substrate for the detection of explosives: Construction strategy and dimensional effect. J. Hazard. Mater. 2020, 387, 121714. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Gong, Z.; Wu, W.; Fan, M.; Wang, D.; Brolo, A.G. Detection of Buried Explosives Using a Surface-Enhanced Raman Scattering (SERS) Substrate Tailored for Miniaturized Spectrometers. ACS Sens. 2020, 5, 2933–2939. [Google Scholar] [CrossRef]
- Verma, A.K.; Soni, R.K. Ultrasensitive surface-enhanced Raman spectroscopy detection of explosive molecules with multibranched silver nanostructures. J. Raman Spectrosc. 2022, 53, 694–708. [Google Scholar] [CrossRef]
- Moldovan, R.; Iacob, B.C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS Detection of Organochlorine Pesticides. Nanomaterials 2021, 11, 304. [Google Scholar] [CrossRef]
- Wang, S.; Sun, B.; Jiang, H.; **, Y.; Feng, J.; An, F.; Wanga, H.; Xu, W. Facile and robust fabrication of hierarchical Au nanorods/Ag nanowire SERS substrates for the sensitive detection of dyes and pesticides. Anal. Methods 2022, 14, 1041–1050. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, G.; Zhang, H.; Zhou, F.; Li, Y.; Cai, W. SERS-based ultrasensitive detection of organophosphorus nerveagents via substrate’s surface modification. J. Hazard. Mater. 2017, 324, 194–202. [Google Scholar] [CrossRef]
- Roldán, M.V.; Scaffardi, L.B.; De Sanctis, O.; Pellegri, N. Optical properties and extinction spectroscopy to characterize the synthesis of amine capped silver nanoparticles. Mater. Chem. Phys. 2008, 112, 984–990. [Google Scholar] [CrossRef]
- Mekonnen, M.L.; Chen, C.H.; Su, W.N.; Hwang, B.J. 3D-functionalized shell isolated Ag nanocubes on a miniaturized flexible platform for sensitive and selective SERS detection of small molecules. Microchem. J. 2018, 142, 305–312. [Google Scholar] [CrossRef]
- Mohan, S.; Oluwafemi, O.S.; Songca, S.P.; Rouxel, D.; Miska, P.; Lewu, F.B.; Kalarikkal, N.; Thomas, S. Nandakumar Kalarikkal and Sabu Thomas, Completely green synthesis of silver nanoparticle decorated MWCNT and its antibacterial and catalytic properties. Pure Appl. Chem. 2016, 88, 71–81. [Google Scholar] [CrossRef]
- Nivethaa, E.A.K.; Narayanan, V.; Stephen, A. Synthesis and spectral characterization of silver embedded chitosan matrix nanocomposite for the selective colorimetric sensing of toxic mercury. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 242–250. [Google Scholar] [CrossRef]
- Gebregeorgis, A.; Bhan, C.; Wilson, O.; Raghavan, D. Characterization of Silver/Bovine Serum Albumin (Ag/BSA) nanoparticles structure: Morphological, compositional, and interaction studies. J. Colloid Interface Sci. 2013, 389, 31–41. [Google Scholar] [CrossRef]
- Sumesh, E.; Bootharaju, M.S.; Anshup; Pradeep, T. A practical silver nanoparticle-based adsorbent for the removal of Hg2+ from water. J. Hazard. Mater. 2011, 189, 450–457. [Google Scholar] [CrossRef]
- Liu, S.; Cui, R.; Ma, Y.; Yu, Q.; Kannegulla, A.; Wu, B.; Fan, H.; Wang, A.X.; Kong, X. Plasmonic cellulose textile fiber from waste paper for BPA sensing by SERS. Biomol. Spectrosc. 2020, 227, 117664. [Google Scholar] [CrossRef]
- Nhat, V.T.; Patra, A.K.; Kim, D. Reductant-Free Synthesis of Silver Nanoparticles by Functionalized Hollow Doughnut Mesoporous Silica Nanoparticles for Preparation of Catalytic Nanoreactor. ChemistrySelect 2018, 3, 1772–1780. [Google Scholar]
- Frattini, A.; Pellegri, N.; Nicastro, D.; de Sanctis, O. Effect of amine groups in the synthesis of Ag nanoparticles using aminosilanes. Mater. Chem. Phys. 2005, 94, 148–152. [Google Scholar] [CrossRef]
- Duan, L.; Wang, P.; Yu, X.; Han, X.; Chen, Y.; Zhao, P.; Lia, D.; Yao, R. The synthesis and characterization of Ag–N dual-doped p-type ZnO: Experiment and theory. Phys. Chem. Chem. Phys. 2014, 16, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.V.; Pellegri, N.; de Sanctis, O. SiO2-K2O-MgO vitreous films doped with erbium and Silver nanoparticles for optical applications. Opt. Mater. 2011, 33, 1921–1926. [Google Scholar] [CrossRef]
- Dhayagude, A.C.; Maiti, N.; Debnath, A.K.; Joshi, S.S.; Kapoor, S. Metal nanoparticle catalyzed charge rearrangement in selenourea probed by surface-enhanced Raman scattering. RSC Adv. 2016, 6, 17405–17414. [Google Scholar] [CrossRef]
- Yang, F.; Long, S.; Zhou, S.; Li, X.; Liu, X.; Gaoa, S.; Kong, Y. Aminosilane decorated carbon template-induced in situ encapsulation of multiple-Ag-cores inside mesoporous hollow silica. RSC Adv. 2016, 6, 30852–30861. [Google Scholar] [CrossRef]
- Chang, C.S.; Wu, K.H.; Hsu, C.Y. Silver nanoparticles embedded cotton swab as surface-enhanced Raman scattering substrate combination of smartphone app for detection of carbofuran residues. Mater. Express 2022, 12, 98–105. [Google Scholar] [CrossRef]
- Lafuente, M.; De Marchi, S.; Urbiztondo, M.; Pastoriza-Santos, I.; Pérez-Juste, I.; Santamaría, J.; Mallada, R.; Pina, M. Plasmonic MOF Thin Films with Raman Internal Standard for Fast and Ultrasensitive SERS Detection of Chemical Warfare Agents in Ambient Air. ACS Sen. 2021, 6, 2241–2251. [Google Scholar]
- Costa, J.C.S.; Ando, R.A.; Sant’Ana, A.C.; Corio, P. Surface-enhanced Raman spectroscopy studies of organophosphorous model molecules and pesticides. Phys. Chem. Chem. Phys. 2012, 14, 15645–15651. [Google Scholar] [CrossRef]
- Yan, F.; Stokes, D.L.; Wabuyele, M.B.; Griffin, G.D.; Vass, A.A.; Vo-Dinh, T. Surface-Enhanced Raman scattering (SERS) detection for chemical and biological agents. In Proceedings of the Biomedical Optics 2004, San Jose, CA, USA, 24–29 January 2004; Volume 5321, pp. 1–7. [Google Scholar]
- Yan, F.; Wabuyele, M.B.; Griffin, G.D.; Vass, A.A.; Vo-Dinh, T. Surface-Enhanced Raman Scattering Detection of Chemical and Biological Agent Simulants. IEEE Sens. J. 2005, 5, 665–670. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Miragliotta, J.A.; Wang, J.; Tyagi, P.; Maddanimath, T.; Gracias, D.H.; Papadakis, S.J. Nanowire-based surface-enhanced Raman spectroscopy (SERS) for chemical warfare simulants. In Proceedings of the SPIE Defense, Security, and Sensing, Baltimore, MD, USA, 23–27 April 2012; Volume 8373, pp. 511–519. [Google Scholar]
- Wang, J.G.; Duan, G.T.; Liu, G.Q.; Li, Y.; Chen, Z.X.; Xu, L.; Cai, W.P. Detection of dimethyl methylphosphonate by thin water filmconfined surface-enhanced Raman scattering method. J. Hazard. Mater. 2016, 303, 94–100. [Google Scholar] [CrossRef]
- Li, T.J.; Wen, B.Y.; Zhang, Y.J.; Zhang, L.; Li, J.F. Au@ZrO2 core-shell nanoparticles as a surface-enhanced Raman scattering substrate for organophosphoruscompounds detection. J. Raman Spectrosc. 2022, 53, 1386–1393. [Google Scholar] [CrossRef]










| Entry | SERS Substrates | Attribute | Sampling (Liquid) | Limit of Detection | Targeted Analytes | Ref. |
|---|---|---|---|---|---|---|
| 1 | AgO | Rigid | Drip Casting | 1 ppm | DMMP CEES DEPA PMP | [33] |
| 2 | Au/Ag | Rigid | Drip Casting | ~0.01 mg/m3 | TPP DMMP Dicrotophos Malathion | [34] |
| 3 | PS/Au/ITO | Rigid | Drip Casting | 0.1 mol/L | DMMP | [35] |
| 4 | Au@ZrO2 | Rigid | Drip Casting | 100 mg/kg | DMMP | [36] |
| 5 | ATS/AgNPs/CS | Flexible | Drip Casting Swabbing Adsorption | 1 g/L | DMMP | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-C.; Chen, H.-R. Application of Cotton Swab–Ag Composite as Flexible Surface-Enhanced Raman Scattering Substrate for DMMP Detection. Molecules 2023, 28, 520. https://doi.org/10.3390/molecules28020520
Huang W-C, Chen H-R. Application of Cotton Swab–Ag Composite as Flexible Surface-Enhanced Raman Scattering Substrate for DMMP Detection. Molecules. 2023; 28(2):520. https://doi.org/10.3390/molecules28020520
Chicago/Turabian StyleHuang, Wen-Chien, and Hong-Ru Chen. 2023. "Application of Cotton Swab–Ag Composite as Flexible Surface-Enhanced Raman Scattering Substrate for DMMP Detection" Molecules 28, no. 2: 520. https://doi.org/10.3390/molecules28020520