Multitemporal Chlorophyll Map** in Pome Fruit Orchards from Remotely Piloted Aircraft Systems
Abstract
:1. Introduction
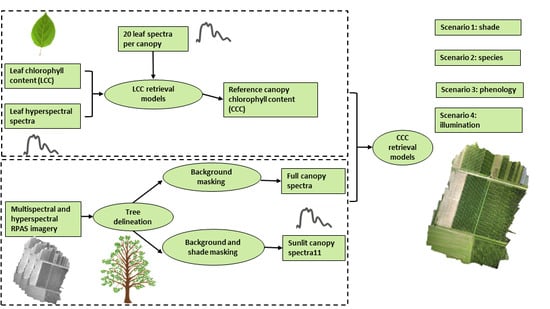
- shadow—we evaluate the CCC retrieval model shade sensitivity by comparing CCC retrieval models extracted from full and sunlit signals from both sensors;
- species—we evaluate the leaf chlorophyll content (LCC) and CCC retrieval model sensitivity of apple and pear species and both species combined from multi- and hyperspectral sensor systems;
- phenology—we evaluate the CCC retrieval model sensitivity to phenological stages by comparing the unitemporal with the multitemporal model performance;
- illumination differences—we evaluate the CCC retrieval model sensitivity to illumination differences by comparing the performance of unitemporal and multitemporal models on image acquisition days with cloudy and clear skies.
2. Materials and Methods
2.1. Study Area
2.2. Remotely Piloted Aircraft System Imagery
2.2.1. Multispectral Imagery
2.2.2. Hyperspectral Imagery
2.3. Leaf Spectral Measurements
2.4. Phenology
2.5. Chlorophyll Retrieval Workflow
2.6. Reference Canopy Chlorophyll Content
2.7. Tree Delineation and Masking
2.8. Retrieval Models
2.8.1. Univariate Retrieval Models
2.8.2. Multivariate Retrieval Models
Linear Models
Non-Linear Models
2.9. Confounding Factors
2.10. Accuracy Assessment
3. Results
3.1. Canopy Shade
3.2. Species Sensitivity
3.2.1. Leaf Chlorophyll Content
3.2.2. Canopy Chlorophyll Content
3.3. Unitemporal versus Multitemporal
3.3.1. Unitemporal
3.3.2. Multitemporal
4. Discussion
4.1. Physiological and Phenological Interpretation of CCC Dynamics
4.2. Confounding Factor Identification, Importance, and Mitigation
4.2.1. Tree Architecture, Shade, and Crop Load Differences between Species
4.2.2. Illumination Variability Caused by Weather
4.3. Multispectral versus Hyperspectral CCC Monitoring in Practice
4.4. Limitations and Recommendations for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| General Characteristics | Pear Orchard | Apple Orchard |
|---|---|---|
| Cultivar | Conference | Golden Delicious |
| Experiment | Drought-nutrient | Chemical thinning with metamitron |
| Rootstock | Quince C | M9 |
| Planting year | 2004 | 2009 |
| Training system | Bush Spindle | Bush Spindle |
| Number of rows | 2 rows | 4 rows |
| Treatments | No nitrogen. Double nitrogen. Drought | 7 different application times with metamitron |
| Total number of plots | 16 plots | 32 plots |
| Experimental trees per plot | 6 | 3 |
| Total number of trees in the experiment | 76 | 96 |
| Total number of monitored trees | 36 | 48 |
| Row distance × tree distance (m) | 3.75 × 1.75 | 3 × 1.5 |
| Mean tree height (m) | 4.18 | 3 |
| Experimental Field | RPAS Multispectral | Acquisition Time RPAS Multispectral | Solar Noon | ASD | Growth Stage |
|---|---|---|---|---|---|
| Apple | 17 May | 01:24-01:36 p.m. | 01:38 p.m. | 23–25 May | Fruit fall after flowering (fruit size up to 10 mm) (BBCH71) |
| 14 June | 09:07-09.21 a.m. | 01:42 p.m. | 12–19 June | Fruit size up to 20 mm, second fruit fall (BBCH 72-73) | |
| 26 July | 10:54-11:07 a.m. | 01:49 p.m. | 26–27 July | Fruit growth and ripening BBCH (73-87) | |
| 29 August | 03:00-03:14 p.m. | 01:49 p.m. | 4–7 September | Fruit ripe for picking (BBCH 87) | |
| 16 October | 08:30-08:43 a.m. | 01:28 p.m. | 12–13 October | Leaf senescence | |
| Pear | 17 May | 03:32-03:50 p.m. | 01:38 p.m. | 30–31 May | Fruit fall after flowering, second fruit fall (BBCH 71-73) |
| 14 June | 02:43-03:03 p.m. | 01:42 p.m. | 20–23 June | Second fruit fall (BBCH 72-73) | |
| 13 July | 11:47 a.m. - 12:12 p.m. | 01:48 p.m. | 18–19 July | Fruit growth and ripening (BBCH 73-87) | |
| 22 August | 10:17-10:33 a.m. | 01:45 p.m. | 14–16 August | Fruit ripe for picking (BBCH 87) | |
| 16 October | 12:54-01:09 p.m. | 01:28 p.m. | 12–13 October | Leaf senescence |
| Experimental Field | RPAS Hyperspectral | Acquisition Time RPAS Hyperspectral | Solar Noon | ASD | Growth Stage |
|---|---|---|---|---|---|
| Apple | 17 May | 02:01-02:07 p.m. | 01:38 p.m. | 23–25 May | Fruit fall after flowering (fruit size up to 10 mm) (BBCH 71) |
| 14 June | 12:08-12:15 p.m. | 01:42 p.m. | 12–19 June | Fruit size up to 20 mm, second fruit fall(BBCH 72-73) | |
| 01:49 p.m. | 26–27 July | Fruit growth and ripening BBCH (73-87) | |||
| 29 August | 06:23-06:32 p.m. | 01:43 p.m. | 4–7 September | Fruit ripe for picking (BBCH 87) | |
| Pear | 17 May | 03:58 – 04:06 p.m. | 01:38 p.m. | 30–31 May | Fruit fall after flowering, second fruit fall (BBCH 71-73) |
| 14 June | 01:17-01:26 p.m. | 01:42 p.m. | 20–23 June | Second fruit fall (BBCH 72-73) | |
| 13 July | 02:20-02:27 p.m. | 01:48 p.m. | 18–19 July | Fruit growth and ripening (BBCH 73-87) | |
| 22 August | 11:24-11:32 a.m. | 01:45 p.m. | 14–16 August | Fruit ripe for picking (BBCH 87) | |
| 16 October | 03:29-03:35 p.m. | 01:28 p.m. | 12–13 October | Leaf senescence |

| Models | Multispectral Full Canopy Spectrum | Multispectral Sunlit Canopy Spectrum | Hyperspectral Full Canopy Spectrum | Hyperspectral Sunlit Canopy Spectrum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VI models | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE |
| Best NDVI | 0.50 (0.05) | 4.83 (0.29) | 20.0% | 0.51 (0.05) | 4.8 (0.30) | 19.9% | 0.53 | 4.41 | 18.3% | 0.56 | 4.28 | 17.7% |
| TCARI/OSAVI | 0.43 (0.07) | 5.18 (0.36) | 21.5% | 0.49 (0.07) | 4.9 (0.36) | 20.3% | 0.02 (0.02) | 6.31 (0.17) | 26.2% | 0.03 (0.03) | 5.63 (0.18) | 23.3% |
| PRI | 0.36 (0.07) | 5.29 (0.32) | 21.9% | 0.51 (0.06) | 4.8 (0.32) | 19.9% | 0.13 (0.06) | 5.91 (0.25) | 24.5% | 0.13 (0.06) | 5.23 (0.23) | 21.7% |
| REIP | 0.41 (0.08) | 5.48 (0.38) | 22.7% | 0.59 (0.05) | 4.4 (0.31) | 18.2% | 0.24 (0.07) | 5.52 (0.28) | 22.9% | 0.22 (0.06) | 4.78 (0.25) | 19.8% |
| Linear multivariate models | ||||||||||||
| RSS | 0.58 (0.11) | 4.43 (0.52) | 18.4% | 0.64 (0.05) | 4.11 (0.31) | 17.0% | 0.63 (0.08) | 3.86 (0.42) | 16.0% | 0.63 (0.08) | 3.89 (0.45) | 16.1% |
| LARS | 0.58 (0.06) | 4.44 (0.26) | 18.4% | 0.64 (0.06) | 4.11 (0.28) | 17.0% | 0.76 (0.06) | 3.08 (0.34) | 12.8% | 0.79 (0.06) | 2.91 (0.30) | 12.1% |
| ENET | 0.58 (0.06) | 4.43 (0.25) | 18.4% | 0.64 (0.06) | 4.11 (0.27) | 17.0% | 0.70 9.00 (0.05) | 2.91 (0.38) | 12.1% | 0.80 (0.06) | 2.83 (0.40) | 11.7% |
| RR | 0.58 (0.05) | 4.43 (0.28) | 18.4% | 0.64 (0.05) | 4.12 (0.29) | 17.1% | 0.79 (0.05) | 2.91 (0.39) | 12.1% | 0.80 (0.04) | 2.83 (0.42) | 11.7% |
| RRVS | 0.58 (0.05) | 4.43 (0.27) | 18.4% | 0.64 (0.05) | 4.12 (0.31) | 17.1% | 0.78 (0.07) | 3.03 (0.33) | 12.6% | 0.79 (0.08) | 2.93 (0.45) | 12.1% |
| PPR | 0.66 (0.07) | 4.00 (0.46) | 16.6% | 0.73 (0.07) | 3.57 (0.48) | 14.8% | 0.59 (0.12) | 4.44 (0.96) | 18.4% | 0.58 (0.15) | 4.48 (1.15) | 18.6% |
| Non-linear multivariate models | ||||||||||||
| RF | 0.70 (0.08) | 3.84 (0.51) | 15.9% | 0.67 (0.09) | 3.93 (0.52) | 16.3% | 0.72 (0.08) | 3.40 (0.50) | 14.1% | 0.74 (0.07) | 3.24 (0.48) | 13.4% |
| TMGA | 0.47 (0.09) | 5.07 (0.43) | 21.0% | 0.54 (0.10) | 4.71 (0.56) | 19.5% | 0.63 (0.11) | 3.89 (0.63) | 16.1% | 0.65 (0.11) | 3.78 (0.64) | 15.7% |
| SGB | 0.68 (0.07) | 3.89 (0.38) | 16.1% | 0.67 (0.08) | 3.91 (0.44) | 16.2% | 0.68 (0.09) | 3.57 (0.50) | 14.8% | 0.73 (0.07) | 3.29 (0.40) | 13.6% |
| SVMR | 0.77 (0.06) | 3.3 (0.40) | 13.7% | 0.72 (0.07) | 3.64 (0.41) | 15.1% | 0.74 (0.06) | 3.21 (0.37) | 13.3% | 0.79 (0.04) | 2.95 (0.29) | 12.2% |
| SVML | 0.58 (0.05) | 4.46 (0.29) | 18.5% | 0.64 (0.05) | 4.14 (0.31) | 17.2% | 0.73 (0.05) | 3.28 (0.32) | 13.6% | 0.77 (0.04) | 3.33 (0.29) | 13.8% |
| GPRR | 0.76 (0.05) | 3.43 (0.37) | 14.2% | 0.72 (0.06) | 3.67 (0.39) | 15.2% | 0.74 (0.05) | 3.28 (0.31) | 13.6% | 0.78 (0.04) | 3.05 (0.28) | 12.6% |
| GPRL | 0.58 (0.05) | 4.45 (0.29) | 18.4% | 0.64 (0.05) | 4.14 (0.31) | 17.2% | 0.73 (0.05) | 3.30 (0.31) | 13.7% | 0.73 (0.06) | 3.31 (0.36) | 13.7% |
| KNN | 0.78 (0.08) | 3.22 (0.58) | 13.3% | 0.72 (0.08) | 3.63 (0.56) | 15.0% | 0.77 (0.07) | 3.02 (0.45) | 12.5% | 0.79 (0.05) | 2.88 (0.37) | 11.9% |
| SBC | 0.53 (0.08) | 4.75 (0.37) | 19.7% | 0.6 (0.08) | 4.38 (0.37) | 18.2% | 0.75 (0.08) | 3.19 (0.53) | 13.2% | 0.75 (0.07) | 3.19 (0.47) | 13.2% |
| Models | Hyperspectral Sunlit Canopy Spectrum | Hyperspectral Sunlit Canopy Spectrum | Hyperspectral Sunlit Canopy Spectrum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Apple | Pear | Pear and Apple | ||||||
| VI models | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE |
| Best NDVI | 0.83 | 2.79 | 12.6% | 0.36 | 4.67 | 19.4% | 0.56 | 4.28 | 17.7% |
| TCARI/OSAVI | 0.25 (0.14) | 5.80 (0.60) | 26.2% | 0.06 (0.13) | 5.76 (0.48) | 23.9% | 0.03 (0.03) | 5.63 (0.18) | 23.3% |
| PRI | 0.43 (0.16) | 5.09 (0.75) | 23.0% | 0.18 (0.10) | 5.30 (0.33) | 22.0% | 0.13 (0.06) | 5.23 (0.23) | 21.7% |
| REIP | 0.55 (0.08) | 4.48 (0.42) | 20.3% | 0.30 (0.12) | 4.94 (0.43) | 20.5% | 0.22 (0.06) | 4.78 (0.25) | 19.8% |
| Linear multivariate models | |||||||||
| RSS | 0.91 (0.02) | 2.03 (0.23) | 9.2% | 0.61 (0.12) | 4.69 (0.67) | 19.4% | 0.63 (0.05) | 3.89 (0.45) | 16.1% |
| LARS | 0.91 (0.05) | 2.24 (0.54) | 10.1% | 0.73 (0.08) | 3.22 (0.35) | 13.3% | 0.79 (0.06) | 2.91 (0.64) | 12.1% |
| ENET | 0.90 (0.05) | 2.10 (0.63) | 9.5% | 0.82 (0.15) | 2.49 (0.53) | 10.3% | 0.80 (0.06) | 2.83 (0.40) | 11.7% |
| RR | 0.91 (0.04) | 2.13 (0.12) | 9.6% | 0.82 (0.13) | 2.49 (0.55) | 10.3% | 0.80 (0.04) | 2.83 (0.42) | 11.7% |
| RRVS | 0.91 (0.04) | 2.03 (0.42) | 9.2% | 0.82 (0.10) | 2.54 (0.42) | 10.5% | 0.79 (0.08) | 2.93 (0.45) | 12.1% |
| PRR | 0.41 (0.17) | 6.39 (0.13) | 28.9% | 0.70 (0.09) | 3.44 (0.54) | 14.3% | 0.58 (0.15) | 4.48 (1.15) | 18.6% |
| Non-linear multivariate models | |||||||||
| RF | 0.90 (0.02) | 2.09 (0.22) | 9.4% | 0.60 (0.12) | 3.66 (0.54) | 15.2% | 0.74 (0.07) | 3.24 (0.48) | 13.4% |
| TMGA | 0.87 (0.09) | 2.28 (0.72) | 10.3% | 0.47 (0.14) | 4.24 (0.64) | 17.6% | 0.65 (0.11) | 3.78 (0.64) | 15.7% |
| SGB | 0.91 (0.02) | 2.04 (0.22) | 9.2% | 0.54 (0.12) | 3.91 (0.53) | 16.2% | 0.73 (0.07) | 3.29 (0.40) | 13.6% |
| SVMR | 0.90 (0.03) | 2.16 (0.22) | 9.8% | 0.57 (0.09) | 3.82 (0.43) | 15.8% | 0.79 (0.04) | 2.95 (0.29) | 12.2% |
| SVML | 0.90 (0.02) | 2.09 (0.26) | 9.4% | 0.78 (0.07) | 2.75 (0.39) | 11.4% | 0.77 (0.04) | 3.33 (0.29) | 13.8% |
| GPRR | 0.87 (0.03) | 2.53 (0.30) | 11.4% | 0.58 (0.09) | 3.92 (0.43) | 16.2% | 0.78 (0.04) | 3.05 (0.28) | 12.6% |
| GPRL | 0.91 (0.02) | 2.05 (0.22) | 9.3% | 0.77 (0.12) | 2.81 (0.55) | 11.6% | 0.73 (0.06) | 3.31 (0.36) | 13.7% |
| KNN | 0.91 (0.02) | 2.05 (0.20) | 9.3% | 0.62 (0.12) | 3.59 (0.55) | 14.9% | 0.79 (0.05) | 2.88 (0.37) | 11.9% |
| SBC | 0.87 (0.03) | 2.53 (0.03) | 11.4% | 0.60 (0.14) | 3.70 (0.71) | 15.3% | 0.75 (0.07) | 3.19 (0.47) | 13.2% |
| Models | Multispectral Sunlit Canopy Spectrum | Multispectral Sunlit Canopy Spectrum | Multispectral Sunlit Canopy Spectrum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Apple | Pear | Pear and Apple | ||||||
| VI models | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE |
| Best NDVI | 0.72 (0.06) | 4.12 (0.41) | 17.2% | 0.62 (0.07) | 3.58 (0.35) | 14.8% | 0.51 (0.05) | 4.80 (0.30) | 19.9% |
| TCARI/OSAVI | 0.74 (0.09) | 3.96 (0.48) | 16.5% | 0.43 (0.09) | 4.32 (0.41) | 17.9% | 0.49 (0.07) | 4.90 (0.36) | 20.3% |
| PRI | 0.72 (0.08) | 4.12 (0.41) | 17.2% | 0.38 (0.03) | 4.55 (0.33) | 18.9% | 0.51 (0.06) | 4.80 (0.32) | 19.9% |
| REIP | 0.66 (0.05) | 4.54 (0.38) | 18.9% | 0.62 (0.09) | 3.56 (0.38) | 14.8% | 0.59 (0.05) | 4.40 (0.31) | 18.2% |
| Linear multivariate models | |||||||||
| RSS | 0.69 (0.07) | 4.33 (0.46) | 18.1% | 0.66 (0.11) | 3.40 (0.49) | 14.1% | 0.64 (0.05) | 4.11 (0.31) | 17.0% |
| LARS | 0.69 (0.07) | 4.33 (0.37) | 18.1% | 0.66 (0.09) | 3.39 (0.34) | 14.0% | 0.64 (0.06) | 4.11 (0.28) | 17.0% |
| ENET | 0.69 (0.06) | 4.33 (0.34) | 18.1% | 0.66 (0.10) | 3.39 (0.38) | 14.0% | 0.64 (0.06) | 4.11 (0.27) | 17.0% |
| RR | 0.69 (0.06) | 4.33 (0.39) | 18.1% | 0.66 (0.09) | 3.39 (0.44) | 14.0% | 0.64 (0.05) | 4.12 (0.29) | 17.1% |
| RRVS | 0.69 (0.06) | 4.33 (0.40) | 18.1% | 0.66 (0.09) | 3.39 (0.42) | 14.0% | 0.64 (0.05) | 4.12 (0.31) | 17.1% |
| PPR | 0.77 (0.07) | 3.67 (0.62) | 15.3% | 0.70 (0.09) | 3.16 (0.50) | 13.1% | 0.73 (0.07) | 3.57 (0.48) | 14.8% |
| Non-linear multivariate models | |||||||||
| RF | 0.68 (0.09) | 4.32 (0.59) | 18.0% | 0.77 (0.08) | 2.78 (0.54) | 11.5% | 0.67 (0.09) | 3.93 (0.52) | 16.3% |
| TMGA | 0.60 (0.13) | 4.97 (0.83) | 20.7% | 0.63 (0.15) | 3.60 (0.75) | 14.9% | 0.54 (0.10) | 4.71 (0.56) | 19.5% |
| SGB | 0.68 (0.08) | 4.35 (0.51) | 18.1% | 0.71 (0.09) | 3.10 (0.53) | 12.8% | 0.67 (0.08) | 3.91 (0.44) | 16.2% |
| SVMR | 0.70 (0.07) | 4.24 (0.47) | 17.7% | 0.80 (0.05) | 2.63 (0.33) | 10.9% | 0.72 (0.07) | 3.64 (0.41) | 15.1% |
| SVML | 0.68 (0.05) | 4.38 (0.38) | 18.3% | 0.65 (0.08) | 2.63 (0.44) | 10.9% | 0.64 (0.05) | 4.14 (0.31) | 17.2% |
| GPRR | 0.70 (0.06) | 4.32 (0.43) | 18.0% | 0.81 (0.05) | 2.66 (0.31) | 11.0% | 0.72 (0.06) | 3.67 (0.39) | 15.2% |
| GPRL | 0.68 (0.05) | 4.35 (0.37) | 18.1% | 0.65 (0.08) | 3.43 (0.42) | 14.2% | 0.64 (0.05) | 4.14 (0.31) | 17.2% |
| KNN | 0.67 (0.09) | 4.45 (0.60) | 18.6% | 0.84 (0.05) | 2.35 (0.35) | 9.7% | 0.72 (0.08) | 3.63 (0.56) | 15.0% |
| SBC | 0.59 (0.09) | 5.00 (0.48) | 20.9% | 0.79 (0.08) | 2.66 (0.42) | 11.0% | 0.60 (0.08) | 4.38 (0.37) | 18.2% |
| Hyperspectral Sunlit | Hyperspectral Sunlit | Hyperspectral Sunlit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Models | Apple | Pear | Pear and Apple | ||||||
| VI models | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE |
| Best NDVI | 0.83 | 2.79 | 12.6% | 0.54 | 2.98 | 15.3% | 0.54 | 3.84 | 17.3% |
| TCARI/OSAVI | 0.25 (0.14) | 5.80 (0.60) | 26.2% | 0.08 (0.12) | 4.23 (0.41) | 21.7% | 0.05 (0.03) | 5.54 (0.25) | 25.0% |
| PRI | 0.43 (0.16) | 5.09 (0.75) | 23.0% | 0.09 (0.08) | 4.13 (0.30) | 21.2% | 0.11 (0.06) | 5.33 (0.25) | 24.0% |
| REIP | 0.55 (0.08) | 4.48 (0.42) | 20.3% | 0.13 (0.09) | 4.05 (0.30) | 20.8% | 0.18 (0.09) | 5.13 (0.32) | 23.1% |
| Linear multivariate models | |||||||||
| RSS | 0.91 (0.02) | 2.03 (0.23) | 9.2% | 0.60 (0.11) | 2.78 (0.48) | 15.9% | 0.73 (0.08) | 3.64 (0.33) | 16.4% |
| LARS | 0.91 (0.05) | 2.24 (0.54) | 10.1% | 0.61 (0.09) | 2.97 (0.41) | 16.8% | 0.80 (0.06) | 2.82 (0.26) | 12.7% |
| ENET | 0.90 (0.05) | 2.10 (0.63) | 9.5% | 0.70 (0.11) | 2.46 (0.65) | 13.8% | 0.80 (0.05) | 2.76 (0.25) | 12.4% |
| RR | 0.91 (0.04) | 2.13 (0.12) | 9.6% | 0.69 (0.11) | 2.49 (0.82) | 13.9% | 0.80 (0.04) | 2.82 (0.23) | 12.7% |
| RRVS | 0.91 (0.04) | 2.03 (0.42) | 9.2% | 0.70 (0.10) | 2.43 (0.43) | 13.9% | 0.78 (0.06) | 3.07 (0.27) | 13.8% |
| PPR | 0.41 (0.17) | 6.39 (0.13) | 28.9% | 0.49 (0.15) | 3.78 (0.72) | 21.8% | 0.67 (0.13) | 4.45 (0.83) | 20.1% |
| Non-linear multivariate models | |||||||||
| RF | 0.90 (0.02) | 2.09 (0.22) | 9.4% | 0.52 (0.12) | 3.04 (0.42) | 16.7% | 0.80 (0.06) | 2.92 (0.33) | 13.2% |
| TMGA | 0.87 (0.09) | 2.28 (0.72) | 10.3% | 0.38 (0.11) | 3.51 (0.44) | 19.7% | 0.76 (0.08) | 3.32 (0.39) | 15.0% |
| SGB | 0.91 (0.02) | 2.04 (0.22) | 9.2% | 0.51 (0.12) | 3.01 (0.42) | 16.1% | 0.80 (0.07) | 2.82 (0.31) | 12.7% |
| SVMR | 0.90 (0.03) | 2.16 (0.22) | 9.8% | 0.53 (0.12) | 2.97 (0.35) | 16.5% | 0.81 (0.05) | 2.79 (0.29) | 12.6% |
| SVML | 0.90 (0.02) | 2.09 (0.26) | 9.4% | 0.71 (0.08) | 2.40 (0.41) | 13.5% | 0.77 (0.06) | 3.07 (0.29) | 13.8% |
| GPRR | 0.87 (0.03) | 2.53 (0.30) | 11.4% | 0.53 (0.12) | 3.06 (0.34) | 17.0% | 0.81 (0.04) | 2.79 (0.26) | 12.6% |
| GPRL | 0.91 (0.02) | 2.05 (0.22) | 9.3% | 0.70 (0.08) | 2.43 (0.42) | 13.9% | 0.77 (0.03) | 3.05 (0.24) | 13.7% |
| KNN | 0.91 (0.02) | 2.05 (0.20) | 9.3% | 0.51 (0.10) | 3.15 (0.35) | 16.7% | 0.82 (0.04) | 2.63 (0.21) | 11.9% |
| SBC | 0.87 (0.03) | 2.53 (0.03) | 11.4% | 0.48 (0.12) | 3.28 (0.48) | 18.3% | 0.78 (0.05) | 3.03 (0.28) | 13.7% |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | BestVI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.21 (0.14) | 0.21 (0.14) | 0.22 (0.13) | 0.23 (0.17) | 0.24 (0.12) | 0.30 (0.26) | 0.26 (0.07) | 0.24 (0.12) | 0.20 (0.06) | 0.22 (0.09) | 0.26 (0.08) | 0.25 (0.12) | 0.15 (0.06) | 0.24 (0.10) | 0.23 (0.67) |
| June |  | 0.20 (0.11) | 0.20 (0.11) | 0.24 (0.13) | 0.17 (0.12) | 0.21 (0.13) | 0.25 (0.08) | 0.22 (0.10) | 0.20 (0.14) | 0.21 (0.07) | 0.24 (0.07) | 0.16 (0.11) | 0.19 (0.15) | 0.13 (0.07) | 0.28 (0.13) | 0.21 (0.12) |
| July |  | 0.14 (0.11) | 0.14 (0.09) | 0.14 (0.09) | 0.19 (0.10) | 0.15 (0.10) | 0.21 (0.10) | 0.16 (0.08) | 0.15 (0.10) | 0.15 (0.09) | 0.15 (0.07) | 0.21 (0.14) | 0.18 (0.11) | 0.16 (0.08) | 0.09 (0.08) | 0.25 (0.13) |
| August |  | 0.19 (0.10) | 0.19 (0.08) | 0.17 (0.09) | 0.16 (0.07) | 0.14 (0.08) | 0.09 (0.14) | 0.17 (0.08) | 0.15 (0.08) | 0.20 (0.09) | 0.20 (0.24) | 0.15 (0.19) | 0.11 (0.10) | 0.23 (0.10) | 0.19 (0.23) | 0.24 (0.11) |
| October |  | 0.26 | 0.26 | 0.29 | 0.21 | 0.27 | 0.31 | 0.15 | 0.26 | 0.12 | 0.20 | 0.16 | 0.27 | 0.12 | 0.14 | 0.37 |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.20 (0.10) | 0.16 (0.08) | 0.18 (0.10) | 0.18 (0.14) | 0.19 (0.10) | 0.21 (0.11) | 0.19 (0.08) | 0.18 (0.10) | 0.20 (0.19) | 0.21 (0.07) | 0.15 (0.19) | 0.15 (0.08) | 0.21 (0.19) | 0.19 (0.07) |
| June |  | 0.16 (0.10) | 0.14 (0.13) | 0.15 (0.11) | 0.17 (0.23) | 0.14 (0.12) | 0.20 (0.08) | 0.19 (0.11) | 0.15 (0.12) | 0.11 (0.15) | 0.25 (0.16) | 0.13 (0.07) | 0.17 (0.11) | 0.13 (0.12) | 0.23 (0.15) |
| July |  | 0.24 | 0.19 | 0.24 | 0.18 | 0.21 | 0.32 | 0.20 | 0.21 | 0.20 | 0.18 | 0.15 | 0.20 | 0.23 | 0.11 |
| August |  | 0.25 (0.10) | 0.19 (0.08) | 0.19 (0.16) | 0.18 (0.19) | 0.19 (0.09) | 0.22 (0.10) | 0.13 (0.12) | 0.18 (0.09) | 0.10 (0.14) | 0.20 (0.10) | 0.12 (0.08) | 0.18 (0.09) | 0.23 (0.14) | 0.15 (0.14) |
| October |  | 0.19 | 0.13 | 0.13 | 0.23 | 0.09 | 0.23 | 0.13 | 0.08 | 0.15 | 0.22 | 0.13 | 0.16 | 0.14 | 0.21 |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | SGB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.28 (0.31) | 0.26 (0.33) | 0.09 (0.14) | 0.59 (0.61) | 0.28 (0.37) | 0.35 (0.45) | 0.49 (0.55) | 0.16 (0.23) | 0.29 (0.32) | 0.5 (0.5) | 0.73 (0.75) | 0.16 (0.59) | 0.37 (0.23) | 0.18 (0.40) | 0.65 (0.18) |
| June |  | 0.6 (0.63) | 0.57 (0.63) | 0.39 (0.42) | 0.76 (0.80) | 0.60 (0.65) | 0.63 (0.66) | 0.83 (0.84) | 0.47 (0.56) | 0.74 (0.74) | 0.85 (0.85) | 0.9 (0.89) | 0.47 (0.81) | 0.79 (0.58) | 0.68 (0.79) | 0.82 (0.64) |
| July |  | 0.03 (<0.01) | 0.02 (<0.01) | <0.01 (<0.01) | 0.38 (0.33) | 0.03 (<0.01) | 0.02 (<0.01) | 0.3 (0.47) | <0.01 (<0.01) | 0.03 (0.05) | 0.31 (0.30) | 0.57 (0.56) | <0.01 (0.32) | 0.03 (0.02) | 0.01 (0.04) | 0.22 (0.02) |
| August |  | 0.06 (0.03) | 0.03 (0.03) | 0.07 (0.02) | 0.71 (0.54) | 0.06 (0.06) | 0.1 (0.13) | 0.9 (0.96) | <0.01 (<0.01) | 0.07 (0.07) | 0.31 (0.59) | 0.5 (0.68) | 0.03 (0.36) | 0.24 (<0.01) | <0.01 (0.26) | 0.44 (<0.01) |
| October |  | 0.02 (0.04) | <0.01 (0.05) | 0.02 (<0.01) | 0.61 (0.72) | 0.02 (0.08) | 0.07 (0.15) | 0.99 (0.99) | 0.01 (<0.01) | 0.16 (0.41) | 0.08 (0.57) | 0.08 (0.85) | <0.01 (0.61) | 0.18 (<0.01) | 0.22 (0.46) | 0.56 (0.11) |
References
- Blackburn, A. Hyperspectral remote sensing of plant pigments. Comp. Biochem. Physiol. Mol. Integr. Physiol. 2006, 143, S147. [Google Scholar] [CrossRef] [PubMed]
- Bolat, I.; Dikilitas, M.; Ercisli, S.; Ikinci, A.; Tonkaz, T. The effect of water stress on some morphological, physiological, and biochemical characteristics and bud success on apple and Quince rootstocks. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Amarante, C.V.T.d.; Steffens, C.A.; Mafra, Á.L.; Albuquerque, J.A. Yield and fruit quality of apple from conventional and organic production systems. Pesqui. Agropecu. Bras. 2008, 43, 333–340. [Google Scholar] [CrossRef]
- Prsa, I.; Stampar, F.; Vodnik, D.; Veberic, R. Influence of nitrogen on leaf chlorophyll content and photosynthesis of ‘Golden Delicious’ apple. Acta Agric. Scand. Sect. B Soil Plant Sci. 2007, 57, 283–289. [Google Scholar] [CrossRef]
- Ma, X.; Feng, J.; Guan, H.; Liu, G. Prediction of chlorophyll content in different light areas of apple tree canopies based on the color characteristics of 3D reconstruction. Remote Sens. 2018, 10, 429. [Google Scholar] [CrossRef]
- Perry, E.M.; Davenport, J.R. Spectral and spatial differences in response of vegetation indices to nitrogen treatments on apple. Comput. Electron. Agric. 2007, 59, 56–65. [Google Scholar] [CrossRef]
- Li, C.; Zhu, X.; Wei, Y.; Cao, S.; Guo, X.; Yu, X.; Chang, C. Estimating apple tree canopy chlorophyll content based on Sentinel-2A remote sensing imaging. Sci. Rep. 2018, 8, 3756. [Google Scholar] [CrossRef] [PubMed]
- Somers, B.; Cools, K.; Delalieux, S.; Stuckens, J.; Van der Zande, D.; Verstraeten, W.W.; Coppin, P. Nonlinear hyperspectral mixture analysis for tree cover estimates in orchards. Remote Sens. Environ. 2009, 113, 1183–1193. [Google Scholar] [CrossRef]
- Van Beek, J.; Tits, L.; Somers, B.; Coppin, P. Stem water potential monitoring in pear orchards through WorldView-2 multispectral imagery. Remote Sens. 2013, 5, 6647–6666. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Lyon, J.G.; Huete, A. Advances in Hyperspectral Remote Sens. of Vegetation and Agricultural Croplands; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Berni, J.A.J.; Zarco-Tejada, P.J.; Suarez, L.; Fereres, E. Thermal and Narrowband Multispectral Remote Sensing for Vegetation Monitoring from an Unmanned Aerial Vehicle. IEEE Trans. Geosci. Remote Sens. 2009, 47, 722–738. [Google Scholar] [CrossRef]
- Duga, A.T.; Ruysen, K.; Dekeyser, D.; Nuyttens, D.; Bylemans, D.; Nicolai, B.M.; Verboven, P. Spray deposition profiles in pome fruit trees: Effects of sprayer design, training system and tree canopy characteristics. Crop Prot. 2015, 67, 200–213. [Google Scholar] [CrossRef]
- Verrelst, J.; Alonso, L.; Camps-Valls, G.; Delegido, J.; Moreno, J. Retrieval of vegetation biophysical parameters using Gaussian process techniques. IEEE Trans. Geosci. Remote Sens. 2012, 50, 1832–1843. [Google Scholar] [CrossRef]
- Degerickx, J.; Roberts, D.; McFadden, J.; Hermy, M.; Somers, B. Urban tree health assessment using airborne hyperspectral and LiDAR imagery. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 26–38. [Google Scholar] [CrossRef] [Green Version]
- Van Beek, J.; Tits, L.; Somers, B.; Deckers, T.; Janssens, P.; Coppin, P. Viewing geometry sensitivity of commonly used vegetation indices towards the estimation of biophysical variables in orchards. J. Imaging 2016, 2, 15. [Google Scholar] [CrossRef]
- **, J.; Wang, Q. Informative bands used by efficient hyperspectral indices to predict leaf biochemical contents are determined by their relative absorptions. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 616–626. [Google Scholar] [CrossRef]
- Féret, J.-B.; Gitelson, A.; Noble, S.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef] [Green Version]
- Cui, R.; Qin, Q.; Yang, N.; Tao, X.; Zhao, S. The optimization of the crop chlorophyll content indices based on a new LAI determination index. In Proceedings of the 2009 IEEE International Geoscience and Remote Sensing Symposium, Cape Town, South Africa, 12–17 July 2009; pp. IV-821–IV-824. [Google Scholar]
- Zou, X.; Hernández-Clemente, R.; Tammeorg, P.; Lizarazo Torres, C.; Stoddard, F.L.; Mäkelä, P.; Pellikka, P.; Mõttus, M. Retrieval of leaf chlorophyll content in field crops using narrow-band indices: Effects of leaf area index and leaf mean tilt angle. Int. J. Remote Sens. 2015, 36, 6031–6055. [Google Scholar] [CrossRef]
- Delalieux, S.; Somers, B.; Hereijgers, S.; Verstraeten, W.; Keulemans, W.; Coppin, P. A near-infrared narrow-waveband ratio to determine Leaf Area Index in orchards. Remote Sens. Environ. 2008, 112, 3762–3772. [Google Scholar] [CrossRef]
- Somers, B.; Delalieux, S.; Verstraeten, W.W.; Eynde, A.V.; Barry, G.H.; Coppin, P. The contribution of the fruit component to the hyperspectral citrus canopy signal. Photogramm. Eng. Remote Sens. 2010, 76, 37–47. [Google Scholar] [CrossRef]
- Aasen, H.; Bolten, A. Multi-temporal high-resolution imaging spectroscopy with hyperspectral 2D imagers—From theory to application. Remote Sens. Environ. 2018, 205, 374–389. [Google Scholar] [CrossRef]
- Kunz, A.; Blanke, M. Effects of global climate change on apple ‘Golden Delicious’ phenology based on 50 years of meteorological and phenological data in Klein-Altendorf. In Proceedings of the IX International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems, Geneva, NY, USA, 4–8 August 2008; pp. 1121–1126. [Google Scholar]
- Lakso, A.N. Apple. In Handbook of Environmental Physiology of Fruit Crops; CRC Press: Boca Raton, FL, USA, 1994; pp. 3–42. [Google Scholar]
- Darbyshire, R.; Farrera, I.; Martinez-Lüscher, J.; Leite, G.B.; Mathieu, V.; El Yaacoubi, A.; Legave, J.-M. A global evaluation of apple flowering phenology models for climate adaptation. Agric. For. Meteorol. 2017, 240, 67–77. [Google Scholar] [CrossRef]
- Verrelst, J.; Camps-Valls, G.; Munoz-Mari, J.; Rivera, J.P.; Veroustraete, F.; Clevers, J.; Moreno, J. Optical remote sensing and the retrieval of terrestrial vegetation bio-geophysical properties—A review. ISPRS J. Photogramm. Remote Sens. 2015, 108, 273–290. [Google Scholar] [CrossRef]
- Stuckens, J.; Somers, B.; Delalieux, S.; Verstraeten, W.; Coppin, P. The impact of common assumptions on canopy radiative transfer simulations: A case study in Citrus orchards. J. Quant. Spectrosc. Radiat. Transf. 2009, 110, 1–21. [Google Scholar] [CrossRef]
- Koetz, B.; Baret, F.; Poilvé, H.; Hill, J. Use of coupled canopy structure dynamic and radiative transfer models to estimate biophysical canopy characteristics. Remote Sens. Environ. 2005, 95, 115–124. [Google Scholar] [CrossRef]
- Verrelst, J.; Munoz, J.; Alonso, L.; Delegido, J.; Rivera, J.P.; Camps-Valls, G.; Moreno, J. Machine learning regression algorithms for biophysical parameter retrieval: Opportunities for Sentinel-2 and -3. Remote Sens. Environ. 2012, 118, 127–139. [Google Scholar] [CrossRef]
- Tu, Y.-H.; Phinn, S.; Johansen, K.; Robson, A. Assessing radiometric correction approaches for multi-spectral UAS imagery for horticultural applications. Remote Sens. 2018, 10, 1684. [Google Scholar] [CrossRef]
- Suomalainen, J.; Anders, N.; Iqbal, S.; Roerink, G.; Franke, J.; Wenting, P.; Hünniger, D.; Bartholomeus, H.; Becker, R.; Kooistra, L. A lightweight hyperspectral map** system and photogrammetric processing chain for unmanned aerial vehicles. Remote Sens. 2014, 6, 11013–11030. [Google Scholar] [CrossRef]
- Pádua, L.; Vanko, J.; Hruška, J.; Adão, T.; Sousa, J.J.; Peres, E.; Morais, R. UAS, sensors, and data processing in agroforestry: A review towards practical applications. Int. J. Remote Sens. 2017, 38, 2349–2391. [Google Scholar] [CrossRef]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J. Hyperspectral imaging: A review on UAV-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Meier, U.; Graf, H.; Hack, H.; Hess, M.; Kennel, W.; Klose, R.; Mappes, D.; Seipp, D.; Stauss, R.; Streif, J. Phanologische Entwicklungsstadien des Kernobstes (Malus domestica Borkh. und Pyrus communis L.), des Steinobstes (Prunus-Arten), der Johannisbeere Ribes-Arten) und der Erdbeere (Fragaria x ananassa. Nachrichtenblatt Dtsch. Pflanzenschutzd. 1994, 46, 141–153. [Google Scholar]
- Belair Hesbania Dataset 2017; KULeuven, V. (Ed.) VITO: Mol, Belgium, 2019. [Google Scholar]
- Lakso, A.; Wünsche, J.; Palmer, J.; Corelli Grappadelli, L. Measurement and modeling of carbon balance of the apple tree. HortScience 1999, 34, 1040–1047. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Delalieux, S.; Somers, B.; Verstraeten, W.; Van Aardt, J.; Keulemans, W.; Coppin, P. Hyperspectral indices to diagnose leaf biotic stress of apple plants, considering leaf phenology. Int. J. Remote Sens. 2009, 30, 1887–1912. [Google Scholar] [CrossRef]
- Rouse, J., Jr.; Haas, R.; Deering, D.; Schell, J.; Harlan, J. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation. [Great Plains Corridor]; NASA: Washington, DC, USA, 1974.
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Gamon, J.; Penuelas, J.; Field, C. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Guyot, G.; Baret, F.; Major, D. High spectral resolution: Determination of spectral shifts between the red and the near infrared. Int. Arch. Photogramm. Remote Sens. 1988, 11, 750–760. [Google Scholar]
- Moser, G.; Zerubia, J.; Serpico, S.B.; Benediktsson, J.A. Mathematical Models and Methods for Remote Sensing Image Analysis: An Introduction. In Mathematical Models for Remote Sens. Image Processing: Models and Methods for the Analysis of 2D Satellite and Aerial Images; Moser, G., Zerubia, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–36. [Google Scholar]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Volume 2. [Google Scholar]
- Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 2008, 28, 1–26. [Google Scholar] [CrossRef]
- Lumley, T. Leaps: Regression Subset Selection; R Package Version 3, Fortran Code by Alan Miller; 2017. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Hastie, T.; Efron, B. Lars: Least Angle Regression, Lasso and Forward Stagewise; R Package Version 1.2; 2013. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Zou, H.; Hastie, T. Elasticnet: Elastic-Net for Sparse Estimation and Sparse PCA.; R Package Version 1.1; 2012. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Zhang, T. Foba: Greedy Variable Selection; R package Version 0.1; 2008. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Grubinger, T.; Zeileis, A.; Pfeiffer, K.-P. Evtree: Evolutionary Learning of Globally Optimal Classification and Regression Trees in R; Working Papers in Economics and Statistics; Universität Innsbruck: Innsbruck, Austria, 2011. [Google Scholar]
- Ridgeway, G. Gbm: Generalized Boosted Regression Models; R Package Version 2.1.3; 2017. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Karatzoglou, A.; Smola, A.; Hornik, K.; Zeileis, A. Kernlab—An S4 Package for Kernel Methods in R. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef]
- Schliep, K.; Hechenbichler, K.; Lizee, A. Kknn: Weighted k-Nearest Neighbors; R Package Version 1.3.1; 2016. Available online: https://cran.r-project.org/ (accessed on 19 June 2019).
- Riza, L.S.; Bergmeir, C.N.; Herrera, F.; Benítez Sánchez, J.M. frbs: Fuzzy Rule-Based Systems for Classification and Regression in R. J. Stat. Softw. 2015, 65, 30. [Google Scholar] [CrossRef]
- Hocking, R.R. A Biometrics invited paper. The analysis and selection of variables in linear regression. Biometrics 1976, 32, 1–49. [Google Scholar] [CrossRef]
- Efron, B.; Hastie, T.; Johnstone, I.; Tibshirani, R. Least angle regression. Ann. Stat. 2004, 32, 407–499. [Google Scholar] [CrossRef] [Green Version]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Zhang, T. Adaptive forward-backward greedy algorithm for learning sparse representations. IEEE Trans. Inf. Theory 2011, 57, 4689–4708. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J.H.; Stuetzle, W. Projection pursuit regression. J. Am. Stat. Assoc. 1981, 76, 817–823. [Google Scholar] [CrossRef]
- Friedman, J.H. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Tuia, D.; Volpi, M.; Verrelst, J.; Camps-Valls, G. Advances in Kernel Machines for Image Classification and Biophysical Parameter Retrieval. In Mathematical Models for Remote Sens. Image Processing: Models and Methods for the Analysis of 2D Satellite and Aerial Images; Moser, G., Zerubia, J., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 399–441. [Google Scholar]
- Fix, E.; Hodges, J.L., Jr. Discriminatory Analysis-Nonparametric Discrimination: Consistency Properties; California University Berkeley: Berkeley, CA, USA, 1951. [Google Scholar]
- Chiu, S. Method and software for extracting fuzzy classification rules by subtractive clustering. In Proceedings of the North American Fuzzy Information Processing, Berkeley, CA, USA, 19–22 June 1996; pp. 461–465. [Google Scholar]
- Kane, V.R.; Gillespie, A.R.; McGaughey, R.; Lutz, J.A.; Ceder, K.; Franklin, J.F. Interpretation and topographic compensation of conifer canopy self-shadowing. Remote Sens. Environ. 2008, 112, 3820–3832. [Google Scholar] [CrossRef]
- Verrelst, J.; Rivera, J.P.; Gitelson, A.; Delegido, J.; Moreno, J.; Camps-Valls, G. Spectral band selection for vegetation properties retrieval using Gaussian processes regression. Int. J. Appl. Earth Obs. Geoinf. 2016, 52, 554–567. [Google Scholar] [CrossRef]
- Ji, R.; Zheng, L.; Deng, X.; Zhang, Y.; Li, M. Forecasting chlorophyll content and moisture of apple leaves in different tree growth period based on spectral reflectance. Trans. Chin. Soc. Agric. Mach. 2014, 45, 269–275. [Google Scholar] [CrossRef]
- Hughes, N.M.; Morley, C.B.; Smith, W.K. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol. 2007, 175, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Archetti, M. Classification of hypotheses on the evolution of autumn colours. Oikos 2009, 118, 328–333. [Google Scholar] [CrossRef]
- Spencer, P.W.; Trrus, J.S. Apple leaf senescence: Leaf disc compared to attached leaf. Plant Physiol. 1973, 51, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, H. Autumnal leaf abscission in apple and pear. Fruit Sci. Rep. 1980, 7, 25–29. [Google Scholar]
- Zarco-Tejada, P.J.; Berni, J.A.; Suárez, L.; Sepulcre-Cantó, G.; Morales, F.; Miller, J.R. Imaging chlorophyll fluorescence with an airborne narrow-band multispectral camera for vegetation stress detection. Remote Sens. Environ. 2009, 113, 1262–1275. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Smith, R.B.; De Pauw, E. Hyperspectral vegetation indices and their relationships with agricultural crop characteristics. Remote Sens. Environ. 2000, 71, 158–182. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2001, 76, 156–172. [Google Scholar] [CrossRef]
- Singha, S.; Baugher, T. Concise Encyclopedia of Temperate Tree Fruit; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Kuuluvainen, T.; Pukkala, T. Simulation of within-tree and between-tree shading of direct radiation in a forest canopy: Effect of crown shape and sun elevation. Ecol. Model. 1989, 49, 89–100. [Google Scholar] [CrossRef]
- Verrelst, J.; Alonso, L.; Caicedo, J.P.R.; Moreno, J.; Camps-Valls, G. Gaussian process retrieval of chlorophyll content from imaging spectroscopy data. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 867–874. [Google Scholar] [CrossRef]
- Filella, I.; Penuelas, J. The red edge position and shape as indicators of plant chlorophyll content, biomass and hydric status. Int. J. Remote Sens. 1994, 15, 1459–1470. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Lichtenthaler, H.K. Detection of red edge position and chlorophyll content by reflectance measurements near 700 nm. J. Plant Physiol. 1996, 148, 501–508. [Google Scholar] [CrossRef]
- Cui, Z.; Gong, G. The effect of machine learning regression algorithms and sample size on individualized behavioral prediction with functional connectivity features. NeuroImage 2018, 178, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Verrelst, J.; Dethier, S.; Rivera, J.P.; Muñoz-Marí, J.; Camps-Valls, G.; Moreno, J. Active learning methods for efficient hybrid biophysical variable retrieval. IEEE Geosci. Remote Sens. Lett. 2016, 13, 1012–1016. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Fernández-Delgado, M.; Sirsat, M.; Cernadas, E.; Alawadi, S.; Barro, S.; Febrero-Bande, M. An extensive experimental survey of regression methods. Neural Netw. 2018. [Google Scholar] [CrossRef] [PubMed]
- Csillik, O.; Cherbini, J.; Johnson, R.; Lyons, A.; Kelly, M. Identification of Citrus Trees from Unmanned Aerial Vehicle Imagery Using Convolutional Neural Networks. Drones 2018, 2, 39. [Google Scholar] [CrossRef]
- Duan, K.; Keerthi, S.S.; Poo, A.N. Evaluation of simple performance measures for tuning SVM hyperparameters. Neurocomputing 2003, 51, 41–59. [Google Scholar] [CrossRef]
- Burkart, A.; Aasen, H.; Alonso, L.; Menz, G.; Bareth, G.; Rascher, U. Angular dependency of hyperspectral measurements over wheat characterized by a novel UAV based goniometer. Remote Sens. 2015, 7, 725–746. [Google Scholar] [CrossRef]
- Roth, L.; Aasen, H.; Walter, A.; Liebisch, F. Extracting leaf area index using viewing geometry effects—A new perspective on high-resolution unmanned aerial system photography. ISPRS-J. Photogramm. Remote Sens. 2018, 141, 161–175. [Google Scholar] [CrossRef]







| VI | Formula | Reference |
|---|---|---|
| NDVI | Rouse et al. (1974) [41] | |
| TCARI | Haboudane et al. (2002) [42] | |
| PRI | Gamon et al. (1992) [43] | |
| REIP | 700 + 40 * | Guyot et al. (1988) [44] |
| Model Class | Model Subclass | Regression Model | Abbreviation | Package | Reference |
|---|---|---|---|---|---|
| Linear | Stepwise linear regression with sequential selection | RSS | leaps | [48] | |
| Linear | Least angle regression | LARS | lars | [49] | |
| Linear | Ridge regression | RR | elasticnet | [50] | |
| Linear | Ridge regression with variable selection | RRVS | foba | [51] | |
| Linear | Linear regression with elastic net | ENET | elasticnet | [50] | |
| Linear | Projection pursuit regression | PPR | MASS | [52] | |
| Non-linear | Decision tree | Random forest | RF | randomForest | [39] |
| Non-linear | Decision tree | Evolutionary algorithm for regression trees | TMGA | evtree | [53] |
| Non-linear | Decision tree | Stochastic gradient boosting | SGB | gbm | [54] |
| Non-linear | Kernel | Support vector machines with linear kernel | SVML | kernlab | [55] |
| Non-linear | Kernel | Support vector machines with radial kernel | SVMR | kernlab | [55] |
| Non-linear | Kernel | Gaussian processes regression with linear kernel | GPRL | kernlab | [55] |
| Non-linear | Kernel | Gaussian processes regression with radial kernel | GPRR | kernlab | [55] |
| Non-linear | Instance based and clustering | K-nearest neighbor | KNN | kknn | [56] |
| Non-linear | Instance based and clustering | Subtractive clustering and fuzzy c-means rules | SBC | frbs | [57] |
| Models | Hyperspectral Leaf Spectrum | Hyperspectral Leaf Spectrum | Hyperspectral Leaf Spectrum | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Apple | Pear | Pear and Apple | ||||||
| VI models | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE | R2 | RMSE | RRMSE |
| Best NDVI | 0.83 (0.05) | 5.53 (0.56) | 8.2% | 0.87 (0.05) | 5.17 (0.68) | 5.7% | 0.84 (0.03) | 5.60 (0.45) | 6.17% |
| TCARI/OSAVI | 0.70 (0.06) | 7.25 (0.72) | 10.8% | 0.61 (0.13) | 9.35 (2.12) | 10.3% | 0.50 (0.07) | 9.88 (0.92) | 10.89% |
| PRI | 0.30 (0.09) | 11.00 (0.97) | 16.4% | 0.53 (0.16) | 9.71 (0.82) | 10.6% | 0.43 (0.11) | 10.46 (0.78) | 11.53% |
| REIP | 0.78 (0.06) | 6.22 (0.65) | 9.3% | 0.61 (0.13) | 12.00 (2.69) | 13.2% | 0.53 (0.13) | 11.63 (3.67) | 12.82% |
| Linear multivariate models | |||||||||
| RSS | 0.82 (0.07) | 5.60 (0.99) | 8.3% | 0.88 (0.05) | 5.01 (1.10) | 5.5% | 0.85 (0.05) | 5.34 (0.78) | 5.9% |
| LARS | 0.79 (0.11) | 6.05 (1.64) | 9.0% | 0.82 (0.14) | 5.18 (3.66) | 5.7% | 0.85 (0.06) | 5.42 (1.46) | 5.9% |
| ENET | 0.81 (0.09) | 5.80 (1.34) | 8.6% | 0.87 (0.09) | 5.24 (2.08) | 5.7% | 0.84 (0.06) | 5.44 (1.04) | 6.0% |
| RR | 0.80 (0.10) | 5.99 (1.51) | 8.9% | 0.87 (0.10) | 5.52 (2.81) | 6.1% | 0.83 (0.06) | 5.58 (1.31) | 6.1% |
| RRVS | 0.81 (0.08) | 5.75 (1.11) | 8.6% | 0.88 (0.05) | 5.02 (1.24) | 5.5% | 0.84 (0.06) | 5.46 (1.02) | 6.0% |
| PPR | 0.51 (0.14) | 11.17 (1.83) | 16.7% | 0.67 (0.23) | 8.91 (3.60) | 9.8% | 0.77 (0.06) | 6.89 (0.06) | 7.6% |
| Non-Linear multivariate models | |||||||||
| RF | 0.77 (0.10) | 6.30 (1.32) | 9.4% | 0.83 (0.10) | 5.94 (1.89) | 6.5% | 0.78 (0.11) | 6.40 (1.14) | 7.0% |
| TMGA | 0.67 (0.11) | 7.77 (1.40) | 11.6% | 0.78 (0.13) | 6.67 (1.52) | 7.3% | 0.69 (0.14) | 7.59 (1.32) | 8.3% |
| SGB | 0.78 (0.07) | 6.18 (1.01) | 9.2% | 0.79 (0.07) | 6.90 (1.01) | 7.6% | 0.78 (0.08) | 6.54 (0.97) | 7.2% |
| SVMR | 0.77 (0.09) | 6.66 (1.34) | 9.9% | 0.69 (0.12) | 7.28 (3.07) | 8.0% | 0.77 (0.09) | 6.96 (1.77) | 7.6% |
| SVML | 0.80 (0.08) | 5.82 (1.08) | 8.7% | 0.87 (0.05) | 5.22 (1.26) | 5.7% | 0.77 (0.05) | 5.48 (0.85) | 6.0% |
| GPRR | 0.70 (0.10) | 7.54 (1.23) | 11.2% | 0.66 (0.11) | 9.48 (2.77) | 10.4% | 0.67 (0.11) | 8.47 (1.91) | 9.3% |
| GPRL | 0.80 (0.08) | 5.87 (1.06) | 8.8% | 0.87 (0.05) | 5.11 (1.04) | 5.6% | 0.84 (0.05) | 5.45 (0.80) | 6.0% |
| KNN | 0.61 (0.15) | 8.29 (1.49) | 12.4% | 0.74 (0.17) | 7.56 (2.08) | 8.3% | 0.64 (0.11) | 8.28 (1.03) | 9.1% |
| SBC | 0.53 (0.5) | 9.33 (1.68) | 13.9% | 0.40 (0.19) | 11.10 (1.87) | 12.2% | 0.51 (0.14) | 9.64 (1.32) | 10.6% |
| Band | All | May | June | July | August | October | |
|---|---|---|---|---|---|---|---|
| Pear | Green | <0.01 | 0.34 | 0.59 | 0.54 | 0.07 | 0.67 |
| Red | 0.06 | 0.25 | 0.55 | 0.23 | 0.09 | 0.78 | |
| Red edge | 0.03 | 0.21 | 0.27 | 0.57 | <0.01 | 0.26 | |
| NIR | 0.03 | 0.27 | 0.3 | 0.6 | <0.01 | 0.4 | |
| Apple | Green | 0.13 | 0.03 | 0.02 | / | 0.19 | / |
| Red | 0.01 | 0.1 | 0.09 | / | 0.19 | / | |
| Red edge | 0.35 | 0.21 | 0.05 | / | 0.08 | / | |
| NIR | 0.27 | 0.27 | <0.01 | / | 0.1 | / |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | BestVI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.00 (0.04) | 0.00 (0.04) | 0.00 (0.04) | 0.01 (0.1) | 0.00 (0.04) | 0.00 (0.04) | 0.10 (0.15) | 0.00 (0.04) | 0.26 (0.01) | 0.07 (0.02) | 0.45 (0.81) | 0.00 (0.04) | 0.60 (0.04) | 0.01 (0.04) | 0 (0.05) |
| June |  | 0.01 (0) | 0.01 (0) | 0.01 (0) | 0.01 (0.1) | 0.01 (0) | 0.01 (0) | 0.04 (0) | 0.01 (0) | 0.14 (0.01) | 0.15 (0.01) | 0.82 (0.35) | 0.01 (0) | 0.47 (0.01) | 0.02 (0.01) | 0 (0) |
| July |  | 0.02 (0) | 0.02 (0) | 0.02 (0) | 0.00 (0.07) | 0.0 (0.02) | 0.02 (0) | 0.07 (0.01) | 0.03 (0) | 0.21 (0.03) | 0.01 (0.01) | 0.71 (0.26) | 0.03 (0) | 0.29 (0.03) | 0.08 (0) | 0.04 (0) |
| August |  | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.01 (0.01) | 0.04 (0.02) | 0.04 (0.02) | 0.16 (0.04) | 0.03 (0.02) | 0.15 (0) | 0.12 (0.07) | 0.70 (0.32) | 0.03 (0.01) | 0.35 (0.03) | 0.00 (0.01) | 0.01 (0.03) |
| October |  | 0.13 | 0.13 | 0.13 | 0.00 | 0.13 | 0.14 | 0.01 | 0.17 | 0.20 | 0.02 | 0.72 | 0.11 | 0.24 | 0.00 | 0.24 |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | SGB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.67 | 0.71 | 0.67 | 0.86 | 0.67 | 0.67 | 0.86 | 0.69 | 0.86 | 0.94 | 0.97 | 0.86 | 0.86 | 0.79 | 0.9 |
| June |  | 0.08 | 0.04 | 0.08 | 0.52 | 0.08 | 0.08 | 0.16 | 0.07 | 0.56 | 0.64 | 0.9 | 0.09 | 0.65 | 0.75 | 0.72 |
| July |  | 0.61 | 0.59 | 0.61 | 0.76 | 0.61 | 0.61 | 0.62 | 0.59 | 0.79 | 0.9 | 0.97 | 0.61 | 0.83 | 0.8 | 0.84 |
| August |  | 0.61 | 0.58 | 0.61 | 0.82 | 0.61 | 0.61 | 0.75 | 0.61 | 0.78 | 0.84 | 0.95 | 0.59 | 0.75 | 0.81 | 0.87 |
| October |  | 0.01 | 0.07 | <0.01 | <0.01 | 0.01 | 0.01 | 0.28 | 0.02 | 0.04 | 0.04 | 0.49 | 0.03 | 0.09 | 0.24 | 0.11 |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.19 (0.31) | 0.60 (0.72) | 0.00 (0) | 0.86 (0.99) | 0.24 (0.05) | 0.24 (0) | 0.32 (0.88) | 0.04 (0.47) | 0.02 (0.30) | 0.16 (0.27) | 0.46 (0.92) | 0.07 (0.5) | 0.07 (0.5) | 0.00 (0) |
| June |  | 0.10 (0) | 0.49 (0.39) | 0.09 (0) | 0.79 (0.39) | 0.14 (0) | 0.12 (0) | 0.29 (0.98) | 0.08 (0.01) | 0.09 (0.20) | 0.02 (0.18) | 0.66 (0.9) | 0.10 (0.04) | 0.15 (0) | 0.08 (0) |
| July |  | 0.05 | 0.62 | 0.06 | 0.76 | 0.10 | 0.12 | 0.48 | 0.00 | 0.07 | 0.04 | 0.52 | 0.00 | 0.07 | 0.00 |
| August |  | 0.04 (0.15) | 0.70 (0.76) | 0.03 (0.04) | 0.56 (0.99) | 0.04 (0.12) | 0.05 (0.05) | 0.88 (0.99) | 0.06 (0.32) | 0.07 (0.06) | 0.02 (0.09) | 0.23 (0.91) | 0.04 (0.38) | 0.15 (0.4) | 0.08 (0) |
| October |  | 0.18 | 0.94 | 0.06 | 0.96 | 0.26 | 0.32 | 0.99 | 0.01 | 0.27 | 0.09 | 0.51 | 0.03 | 0.27 | 0.01 |
| Weather | ENET | LARS | RSS | PPR | RR | RRVS | SBC | GPRL | GPRR | KNN | RF | SVML | SVMR | TMGA | SGB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| May |  | 0.24 | 0.56 | 0.09 | 0.79 | 0.31 | 0.03 | 0.85 | 0.17 | 0.04 | 0.06 | 0.45 | 0.22 | 0.14 | 0 | 0.79 |
| June |  | 0.08 | 0.55 | <0.01 | 0.84 | 0.14 | 0.56 | 0.56 | 0.03 | 0.06 | 0.12 | 0.73 | 0.06 | 0.18 | 0.06 | 0.86 |
| July |  | 0.11 | 0.61 | <0.01 | 0.76 | 0.16 | 0.03 | 0.61 | 0.03 | 0.15 | 0.34 | 0.61 | 0.02 | 0.07 | 0.01 | 0.66 |
| October |  | 0.29 | 0.92 | <0.01 | 0.95 | 0.38 | 0.08 | 0.99 | 0.08 | 0.63 | 0.5 | 0.93 | 0.06 | 0.74 | 0.07 | 0.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanbrabant, Y.; Tits, L.; Delalieux, S.; Pauly, K.; Verjans, W.; Somers, B. Multitemporal Chlorophyll Map** in Pome Fruit Orchards from Remotely Piloted Aircraft Systems. Remote Sens. 2019, 11, 1468. https://doi.org/10.3390/rs11121468
Vanbrabant Y, Tits L, Delalieux S, Pauly K, Verjans W, Somers B. Multitemporal Chlorophyll Map** in Pome Fruit Orchards from Remotely Piloted Aircraft Systems. Remote Sensing. 2019; 11(12):1468. https://doi.org/10.3390/rs11121468
Chicago/Turabian StyleVanbrabant, Yasmin, Laurent Tits, Stephanie Delalieux, Klaas Pauly, Wim Verjans, and Ben Somers. 2019. "Multitemporal Chlorophyll Map** in Pome Fruit Orchards from Remotely Piloted Aircraft Systems" Remote Sensing 11, no. 12: 1468. https://doi.org/10.3390/rs11121468