Preparation of CdS Nanoparticles-TiO2 Nanorod Hererojunction and Their High-Performance Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Surface Morphology Characterization
2.2. Crystal Structure Characterization
2.3. Test of Absorption Performance
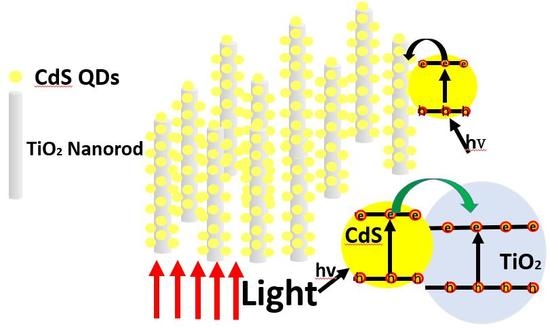
2.4. Photocurrent Test
2.5. BET Test
2.6. Photocatalytic Performance
3. Materials and Methods
3.1. Preparation of the Composite of TiO2 Nanorods and CdS Nanoparticles
3.1.1. Equipment and Reagents
3.1.2. Experimental Procedure
3.2. Characterization of Physical Performance
3.3. Characterization of the Photocatalytic Activities of the Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Murgolo, S.; Franz, S.; Arab, H.; Bestetti, M.; Falletta, E.; Mascolo, G. Degradation of emerging organic pollutants in wastewater effluents by electrochemical photocatalysis on nanostructured TiO2 meshes. Water Res. 2019, 164, 114920. [Google Scholar] [CrossRef] [PubMed]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Cabrera-Rein, A.; Martínez-Piernas, A.B.; Yannis, B.; Xekoukoulotakis, N.P.; Agüera, A.; Pérezbd, J.A. TiO2 photocatalysis under natural solar radiation for the degradation of the carbapenem antibiotics imipenem and meropenem in aqueous solutions at pilot plant scale. Water Res. 2019, 166, 115037. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Prasad, K.; San**es, R.; Schmid, P.E.; Levy, F. Electrical and optical properties of TiO2 anatase thin films. J. Appl. Phys. 1994, 75, 2042–2047. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.; Stout, J.; Gole, J.L. Enhanced nitrogen do** in TiO2 nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Antonelli, D.M.; Ying, J.Y. Synthesis of hexagonally packed mesoporous TiO2 by a modified sol-gel method. Angew. Chem. Int. Edit. 1995, 34, 2014–2017. [Google Scholar] [CrossRef]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Bozic, A.L. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2020, 382, 122826. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, S.L.; Ding, J.; Meng, Y.H.; Zhong, Q.; Kong, D.S.; Gu, C.J. Effect of adsorption properties of phosphorus-doped TiO2 nanotubes on photocatalytic NO removal. J. Colloid. Interf. Sci. 2019, 553, 647–654. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhao, Y.C.; Yang, J.P.; Zhang, J.Y.; Zheng, C.G. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal conbustion flue gas. Chem. Eng. J. 2018, 348, 618–629. [Google Scholar] [CrossRef]
- Kazemi, M.L.; Sui, R.H.; Clark, P.D.; Marriott, R.A. Catalytic combustion of Claus tail gas: Oxidation of sulfur species and CO using gold supported on lanthanide-modified TiO2. Appl. Cataly A Gen. 2019, 578, 117256. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, Y.N.; Wang, L.; Zhang, Y.L.; Wang, D.G.; Yang, M.; Yang, J.H.; Zhang, B.Y.; Jiang, Z.X.; Li, C. Highly efficient photocatalytic oxidation of sulfur-containing organic compounds and dyes on TiO2 with dual cocatalysts Pt and RuO2. Appl. Catal. B Environ. 2012, 127, 363–370. [Google Scholar] [CrossRef]
- Pei, X.L.; Jiang, C.J.; Chen, W. Enhanced hydrolysis of 1,1,2,2-tetrachloroethane by multi-walled carbon nanotube/TiO2 nanocomposites: The synergistic effect. Environ. Pollut. 2019, 255, 113211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Yang, C.Z.; Chen, A.; Pu, W.H.; Gong, J.Y. Influence of yolk-shell Au@TiO2 structure induced photocatalytic activity towards gaseous pollutant degradation under visible light. Appl. Catal. B Environ. 2019, 251, 57–65. [Google Scholar] [CrossRef]
- Elfalleh, W.; Assadi, A.A.; Bouzaza, A.; Wolbert, D.; Kiwi, J.; Rtimi, S. Innovative and stable TiO2 supported catalytic surfaces removing aldehydes under UV-light irradiation. J. Photoch. Photobio A Chem. 2017, 343, 96–102. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, M.; Tian, M.; Zhao, W.F. In situ hydrothermal preparation and photocatalytic desulfurization performance of graphene wrapped TiO2 composites. J. Solid. State. Chem. 2019, 279, 120953. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Dionísio, A. Self-cleaning property of mortars with TiO2 addition using real diesel exhaust soot. J. Clean. Prod. 2017, 161, 850–859. [Google Scholar] [CrossRef]
- Chai, W.M.; Zhu, W.D.; Chen, D.D.; Chen, D.Z.; ** on key optoelectrical properties of CdS thin films prepared using spray pyrolysis technique for high-performance photodetector applications. Ceram. Int. 2020, 46, 4652–4663. [Google Scholar] [CrossRef]
- Sun, C.Y.; Xu, Q.H.; **e, Y.; Ling, Y.; Jiao, J.L.; Zhu, H.H.; Zhao, J.S.; Liu, X.M.; Hu, B.; Zhou, D. High-efficient one-pot synthesis of carbon quantum dots decorating Bi2MoO6 nanosheets heterostructure with enhanced visible-light photocatalytic properties. J. Alloys Compd. 2017, 723, 333–344. [Google Scholar] [CrossRef]
- Fu, H.H.; Yang, L.M.; Hu, D.S.; Yu, c.; Ling, Y.; **e, Y.; Li, S.Q.; Zhao, J.S. Titanium dioxide nano-heterostructure with nanoparticles decorating nanowires for high-performance photocatalysis. Int. J. Hydrogen Energ. 2018, 43, 10359–10367. [Google Scholar] [CrossRef]
- Tu, H.; Li, D.; Yi, Y.; Liu, R.; Wu, Y.; Dong, X.Y.; Shi, X.W.; Deng, H.B. Incorporation of rectorite into porous polycaprolactone/TiO2 nanofibrous mats for enhancing photocatalysis properties towards organic dye pollution. Compos. Commun. 2019, 15, 58–63. [Google Scholar] [CrossRef]
- Wu, J.; **e, Y.; Ling, Y.; Dong, Y.Y.; Li, J.; Li, S.Q.; Zhao, J.S. Synthesis of flower-like g-C3N4/BiOBr and enhancement of the activity for the degradation of bisphenol A under visible light irradiation. Front. Chem. 2019, 7, 649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.Y.; Jiang, R.; **ao, L.; Liu, L.; Cao, C.H.; Zeng, G.M. CdS nanocrystals/TiO2/crosslinked chitosan composite: Facile preparation, characterization and adsorption-photocatalytic properties. Appl. Surf. Sci. 2013, 273, 661–669. [Google Scholar] [CrossRef]










| Project Type Size | BET Surface Area (m2/g) | Pore Size (nm) |
|---|---|---|
| TiO2(40%)@CdS | 61.4 | 35.5 |
| TiO2(30%)@CdS | 53.6 | 42.7 |
| TiO2(40%)@CdS | 107.5 | 14.6 |
| TiO2(50%)@CdS | 86.5 | 25.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Zeng, D.; **e, Y.; Zhang, F.; Rao, S.; Wang, F.; Zhao, J.; Zhang, J.; Wang, L. Preparation of CdS Nanoparticles-TiO2 Nanorod Hererojunction and Their High-Performance Photocatalytic Activity. Catalysts 2020, 10, 441. https://doi.org/10.3390/catal10040441
Song J, Zeng D, **e Y, Zhang F, Rao S, Wang F, Zhao J, Zhang J, Wang L. Preparation of CdS Nanoparticles-TiO2 Nanorod Hererojunction and Their High-Performance Photocatalytic Activity. Catalysts. 2020; 10(4):441. https://doi.org/10.3390/catal10040441
Chicago/Turabian StyleSong, Jianhua, Dedong Zeng, Yu **e, Fayun Zhang, Shenli Rao, Fahui Wang, **sheng Zhao, **bing Zhang, and Lei Wang. 2020. "Preparation of CdS Nanoparticles-TiO2 Nanorod Hererojunction and Their High-Performance Photocatalytic Activity" Catalysts 10, no. 4: 441. https://doi.org/10.3390/catal10040441