2.1. Catalytic Oxidation of Chlorobenzene
The catalytic oxidation of chlorobenzene was conducted over different Ru-based samples, such as 1Ru/TiO
2(P25), 1Ru/ZrO
2, 1Ru/γ-Al
2O
3, and 1Ru/SiO
2. The results are summarized in
Figure 1a. Among those catalysts, 1Ru/TiO
2(P25) contributed the lowest complete oxidation temperature at 280 °C, revealing that commercial P25 TiO
2 was the best support for Ru catalysts in chlorobenzene oxidation, and it was believed that the rutile phase played an important role in the P25 support due to the similar interplanar lattice spacings for RuO
2 and rutile (110) of TiO
2. Besides, the intrinsic physicochemical properties of the supports are also of great concern on their catalytic performance. Considering that P25 exists as a mixed phase containing the anatase and rutile phases, 1Ru/TiO
2(P25), 1Ru/TiO
2(Anatase), and 1Ru/TiO
2(Rutile) were prepared and compared in chlorobenzene oxidation. As shown in
Figure 1b, 1Ru/TiO
2(P25) was still the most active catalyst. 1Ru/TiO
2(Rutile) gave far lower catalytic activity than the other two samples, and its conversion was below 50% at 350 °C. Similar phenomenon has been reported in our previous work on the catalytic oxidation of trichloroethylene over Ru/TiO
2 catalysts [
33]. RuO
2 was commonly well-dispersed on rutile TiO
2, whereas RuO
2 sintering occurred on Ru/TiO
2(P25) and Ru/TiO
2(Anatase). However, particle size effect was often observed for noble metal catalysts. In this catalytic system, Ru/TiO
2(P25) showed the highest activity than Ru/TiO
2(Rutile) and Ru/TiO
2(Anatase) according to the combined effects of dispersity and particle size effect.
Bimetallic catalysts commonly showed higher activity than monometallic catalysts due to the synergistic effect. To further increase in the catalytic activity of 1Ru/TiO
2 materials, ceria oxides were introduced into the Ru catalytic system (
Figure 2). The T
90 of 1Ru-5Ce/TiO
2(Rutile), 1Ru-5Ce/TiO
2(P25), and 1Ru-5Ce/TiO
2(Anatase) were 279, 283, and 290 °C, respectively. Surprisingly, the catalytic activity of 1Ru-5Ce/TiO
2(Rutile) was dramatically improved. However, the catalytic activity of P25 supported catalysts decreased with Ce addition. 1Ru-5Ce/TiO
2(Anatase) showed slight catalytic improvement in comparison to 1Ru/TiO
2(Anatase). It could be concluded that the support crystal phase plays an important role in Ru-catalyzed chlorobenzene oxidation, which is consistent with previous reports.
The chlorobenzene consumption rates for 1Ru/TiO
2 and 1Ru-5Ce/TiO
2 catalysts were compared at 280 °C (
Figure 3). It is noteworthy that 1Ru/TiO
2(P25) contributed the highest chlorobenzene consumption rate (0.37 μmol/(g s)) as compared to the other samples, demonstrating that the monometallic Ru catalytic system was appropriate for P25 support. Obviously, 1Ru-5Ce/TiO
2(Rutile) gave a far higher chlorobenzene consumption rate (0.34 μmol/(g s)) than that of 1Ru/TiO
2(Rutile) (0.01 μmol/(g s)), revealing that the Ru-Ce bimetallic catalytic system was very suitable for rutile TiO
2 support. Considering the lower cost of rutile TiO
2 as compared to P25, it is very important to conduct systematic studies for rutile TiO
2 supported Ru-Ce bimetallic catalysts. It is noteworthy that Ru-5Ce/TiO
2(Rutile) showed comparable catalytic performance to Ru/TiO
2(P25), and the RuO
2 active species could be highly trapped and stabilized by TiO
2(Rutile) and CeO
2, which was proved in the HAADF-STEM images.
To evaluate catalytic stability, on-stream chlorobenzene oxidation experiments were carried out for 1Ru-5Ce/TiO
2(P25), 1Ru-5Ce/TiO
2(Rutile), and 1Ru-5Ce/TiO
2(Anatase). As shown in
Figure 4a, 1Ru-5Ce/TiO
2(Rutile) gave best catalytic performance at 280 °C. For 1Ru-5Ce/TiO
2(Rutile) and 1Ru-5Ce/TiO
2(P25), the chlorobenzene conversion stabilized at approximately 91% and 86%, respectively. Although 1Ru-5Ce/TiO
2(Anatase) activity showed an increasing trend over time, its catalytic activity was far lower than other two materials. Besides, light-off curves with cycle experiments were conducted. As shown in
Figure 4b, the catalytic activity slightly decreased within the fourth runs. However, the catalytic activity was then stabilized, and the conversion in 16th run was basically comparable to the 4th run, indicating excellent stability for 1Ru-5Ce/TiO
2(Rutile).
For VOCs purification applications, CO
2 selectivity was also an important factor for the catalyst. In the catalytic oxidation of chlorobenzene over 1Ru/TiO
2(Rutile) and 1Ru-5Ce/TiO
2(Rutile), organic byproducts such as multi-chlorinated benzenes were basically not observed. Hence, the CO
2 selectivity was calculated based on CO
2 and CO. As shown in
Figure 5, 1Ru-5Ce/TiO
2(Rutile) gave excellent CO
2 selectivity (98–100%), which was apparently better than 1Ru/TiO
2(Rutile), revealing that Ru-Ce bimetallic catalytic system was beneficial to enhencing CO
2 selectivity.
2.2. Catalyst Characterization
The XRD patterns of Ru-based monometallic and bimetallic catalysts were collected. As shown in
Figure 6a, 1Ru/TiO
2(P25) and 1Ru/ZrO
2 showed no peaks ascribed to ruthenium species, possibly due to being well dispersed. For 1Ru/SiO
2 and 1Ru/γ-Al
2O
3, peaks attributable to RuO
2 were observed at 28.0°, 35.1°, and 54.3°, revealing that RuO
2 was easily aggregated on the SiO
2 and γ-Al
2O
3 surface, suggesting that this could be the main reason for their poor catalytic activities. In
Figure 6b, 1Ru/TiO
2 and 1Ru-5Ce/TiO
2 showed similar XRD patterns, and no ceria species were observed.
XPS spectra for the catalysts 1Ru/TiO
2 and 1Ru-5Ce/TiO
2 were obtained; the Ru 3d and O 1s spectra are illustrated in
Figure 7. As shown in
Figure 7a, the supports of P25 and anatase gave the main peaks at similar a binding energy, whereas the rutile TiO
2 support showed a major peak at a higher binding energy (BE) value. The main peaks showed apparent shifts to the high BE value after the addition of the CeO
2. Considering that the assignments and definitions of Ru peaks were inconsistent in previous studies, and apparent shifts were observed, Ru 3d spectra were not de-convoluted in this research. The XPS data were summarized in
Table 1. It could be seen that 1Ru/TiO
2(Rutile) contributed the highest Ru content on the catalyst surface, whereas giving the lowest catalytic activity, revealing that the dispersion was not the only factor influencing the catalytic performance. As illustrated in
Figure 7b, O 1s were de-convoluted into two peaks at 529.8 and 531.8 eV, ascribed to O
latt (lattice oxygen) and O
ads (adsorbed oxygen, e.g., O
2−, O
22−, or O
−), respectively. It was generally believed that the electrophilic O
ads species play an important role in VOCs oxidation. For the three 1Ru-5Ce/TiO
2 catalysts, 1Ru-5Ce/TiO
2(Rutile) contributed the highest Ru/Ce ratio (0.3) and O
ads /O
latt ratio (0.2) possibly due to the synergistic effect between Ru and Ce.
TEM and high resolution transmission electron microscopy (HRTEM) characterizations were conducted to identify the morphology of 1Ru/TiO
2(Rutile) and 1Ru-5Ce/TiO
2(Rutile). As shown in
Figure 8a,b, RuO
2 species were not observed on the surface of 1Ru/TiO
2(Rutile), possibly due to its thin layer structure which usually showed a low contrast in TEM images. However, for 1Ru-5Ce/TiO
2(Rutile), abundant RuO
2 nanoparticles were observed (
Figure 8c), revealing that RuO
2 nanoparticles were easily formed in the presence of CeO
2. Interplanar lattice spacings for RuO
2 and rutile (110) of TiO
2 were observed, and it could be seen that they exhibit similar lattice spacing values. Hence, RuO
2 nanoparticles could be highly stabilized by TiO
2(Rutile), leading to higher catalytic activity and stability than other bimetallic materials.
To further explore the distributions of monometallic Ru and bimetallic Ru-Ce species, HAADF-STEM and STEM-energy dispersive X-ray spectroscopy (STEM-EDS) map** images were collected for 1Ru/TiO
2(Rutile) and 1Ru-5Ce/TiO
2(Rutile). As shown in
Figure 9, the distributions for Ru (green), Ti (red), and Ce (purple) are presented. For 1Ru/TiO
2(Rutile), Ru and Ti showed a similar distribution, indicating that RuO
2 was highly dispersed on the rutile TiO
2 support due to their similar crystal lattice spacing values. However, the highly dispersed catalyst gave poor catalytic performance, revealing that dispersion was not the major factor for the catalytic activity, which was different from the other catalytic systems. For 1Ru-5Ce/TiO
2(Rutile), it was interestingly observed that Ru species were surrounded by CeO
2 and therefore forming the trapped RuO
2. In consideration of its high activity, it was proposed that this unique structure greatly facilitated catalytic activity and stability.
In order to research the reducibility of the catalysts, H
2-TPR profiles of 1Ru-5Ce/TiO
2(Rutile), 1Ru/TiO
2(Rutile), and 5Ce/TiO
2(Rutile) were collected and summarized in
Figure 10. A single reduction peak at 150 °C for 1Ru/TiO
2(Rutile), which was ascribed to the reduction of RuO
2 to metallic Ru, and the actual H
2 consumption was 0.187 mmol/g, which was close to the theoretical value (0.198 mmol/g) calculated by assuming that all Ru atoms in the catalysts existed as Ru
4+. For 1Ru-5Ce/TiO
2(Rutile), a broad peak at 128 °C was observed assigned to the overlap** of the reduction of RuO
2 and the active oxygen species in CeO
2. Apparently, the reducibility was enhanced in the Ru-Ce bimetallic catalyst, suggesting a synergistic effect between Ru and Ce in 1Ru-5Ce/TiO
2(Rutile). The hydrogen spillover effect was commonly observed in the low temperature reduction of a catalyst composed of transition metal oxide and noble metal [
49].
2.3. In Situ FTIR Studies and Reaction Mechanism
To further investigate the reaction mechanism of chlorobenzene oxidation over 1Ru-5Ce/TiO
2(Rutile), in situ FTIR spectra were collected. As shown in
Figure 11, the band at 1892 cm
−1 was tentatively ascribed to the trace maleic anhydride coordinated to Ru
δ+ at the corner sites of the RuO
x clusters according to the previous research [
49]. The band at 1598 cm
−1 was ascribed to the phenolate species. The band at 1731 cm
−1 was assigned to the C=O from quinone or other ketone species [
23]. The bands between 1568–1526 cm
−1 were due to the COO-antisymmetric stretching vibration of (chlorinated)-maleate and acetates, and the band at 1404 cm
−1 was attributable to the COO-symmetric stretching vibrations of (chlorinated)-maleate and acetates. The band at 1367 cm
−1 was assigned to the –CH
2–stretching vibration of (chlorinated)-acetates.
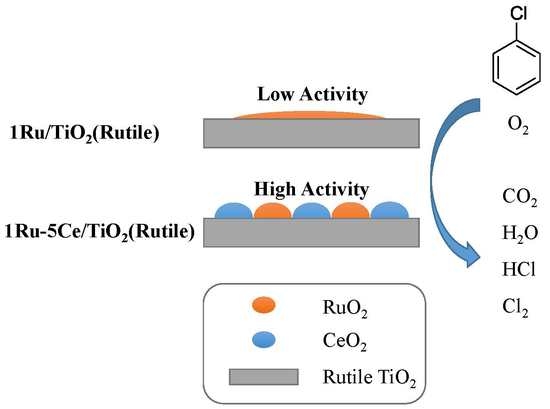
Accordingly, the reaction mechanism was proposed. As shown in
Figure 12, in comparison to 1Ru/TiO
2(Rutile), 1Ru-5Ce/TiO
2(Rutile) contributed a much higher catalytic efficiency due to its trapped RuO
2 structure caused by CeO
2. During the oxidation, CeO
2 also play an important role for affording the active oxygen species, which facilitated the oxidation of chlorobenzene over RuO
2 centers. For 1Ru-5Ce/TiO
2(Rutile) (A), chlorobenzene (B) was firstly oxidized into phenolate species (C). Then, the phenolate species are further oxidized into o-benzoquinone (D) and p-benzoquinone (E). Subsequently, small organic intermediates are generated through the ring-opening process, and the intermediates are easily chlorinated by the chlorine released from catalyst surface (C), leading to (chlorinated)-maleate (F) and acetates (G). Finally, the intermediates are decomposed into CO
2, H
2O, HCl, and Cl
2.