Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ni, H.J.; Liu, J.G.; Wang, Z.H.; Yang, S.Y. A review on colorless and optically transparent polyimide films: Chemistry, process and engineering applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Zhang, X.M.; Song, Y.Z.; Liu, J.G.; Yang, S.Y. Synthesis and properties of cost-effective light-color and highly transparent polyimide films from fluorine-containing tetralin dianhydride and aromatic diamines. J. Photopolym. Sci. Technol. 2016, 29, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.L.; Yen, H.J.; Liou, G.S. Highly transparent polyimide hybrids for optoelectronic applications. React. Funct. Polym. 2016, 108, 2–30. [Google Scholar] [CrossRef]
- Bae, W.J.; Kovalev, M.K.; Kalinina, F.; Kim, M.; Cho, C.K. Towards colorless polyimide/silica hybrids for flexible substrates. Polymer 2016, 105, 124–132. [Google Scholar] [CrossRef]
- Spechler, J.A.; Koh, T.W.; Herb, J.T.; Rand, B.P.; Arnold, C.B. A transparent, smooth, thermally robust, conductive polyimide for flexible electronics. Adv. Funct. Mater. 2015, 48, 7428–7434. [Google Scholar] [CrossRef]
- Kim, Y.M.; Song, C.H.; Kwak, M.G.; Ju, B.K.; Kim, J.W. Flexible touch sensor with finely patterned Ag nanowires buried at the surface of a colorless polyimide film. RSC Adv. 2015, 5, 42500–42505. [Google Scholar] [CrossRef]
- Kang, S.B.; Kim, H.J.; Noh, Y.J.; Na, S.I.; Kim, H.K. Face-to-face transferred multicrystalline ITO films on colorless polyimide substrates for flexible organic solar cells. Nano Energy 2015, 11, 179–188. [Google Scholar] [CrossRef]
- Choi, S.J.; Kim, S.J.; Jang, J.S.; Lee, J.H.; Kim, I.D. Silver nanowire embedded colorless polyimide heater for wearable chemical sensors: Improved reversible reaction kinetics of optically reduced graphene oxide. Small 2016, 12, 5826–5835. [Google Scholar] [CrossRef]
- Wu, X.; Liu, J.G.; Jiang, G.L.; Zhang, Y.; Guo, C.Y.; Zhang, Y.J.; Qi, L.; Zhang, X.M. Highly transparent preimidized semi-alicyclic polyimide varnishes with low curing temperatures and desirable processing viscosities at high solid contents: Preparation and applications for LED chip passivation. J. Mater. Sci. Mater. Electron. 2019, 30, 549–560. [Google Scholar] [CrossRef]
- Choi, I.H.; Chang, J.H. Colorless polyimide nanocomposite films containing hexafluoroisopropylidene group. Polym. Adv. Technol. 2011, 22, 682–689. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, S.Y.; Chung, I.S. Soluble and transparent polyimides from unsymmetrical diamine containing two trifluoromethyl groups. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4413–4422. [Google Scholar] [CrossRef]
- Tapaswi, P.K.; Choi, M.C.; Nagappan, S.; Ha, C.S. Synthesis and characterization of highly transparent and hydrophobic fluorinated polyimides derived from perfluorodecylthio substituted diamine monomers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 479–488. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hirano, D.; Fujii, M.; Haga, M.; Takezawa, E.; Yamaguchi, S.; Ishikawa, A.; Kagayama, T. Solution-processable colorless polyimides derived from hydrogenated pyromellitic dianhydride with controlled steric structure. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 575–592. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horiuchi, M.; Kumakura, K.; Koyama, J. Colorless polyimides with low coefficient of thermal expansion derived from alkyl-substituted cyclobutanetetracarboxylic dianhydrides. Polym. Int. 2014, 63, 486–500. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Fujiwara, Y.; Kitaoka, T.; Oishi, Y.; Shibasaki, Y. Synthesis of highly transparent poly (amide–imide) s based on trimellitic acid and dependence of thermal properties on monomer sequence. React. Funct. Polym. 2016, 108, 78–85. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of solution-processable, optically transparent polyimides with ultra-low linear coefficients of thermal expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Zhang, X.M.; Liu, J.G.; Yang, S.Y. A review on recent progress of R&D for high-temperature resistant polymer dielectrics and their applications in electrical and electronic insulation. Rev. Adv. Mater. Sci. 2016, 46, 22–38. [Google Scholar]
- Fan, H.B.; Yang, R.J. Flame-retardant polyimide cross-linked with polyhedral oligomeric octa (aminophenyl) silsesquioxane. Ind. Eng. Chem. Res. 2013, 52, 2493–2500. [Google Scholar] [CrossRef]
- Lin, C.H.; Chang, S.L.; Cheng, P.W. Soluble high-Tg polyetherimides with good flame retardancy based on an asymmetric phosphinated etherdiamine. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 1331–1340. [Google Scholar] [CrossRef]
- Ando, S.; Matsuura, T.; Sasaki, S. Coloration of aromatic polyimides and electronic properties of their source materials. Polym. J. 1997, 29, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tsai, C.Y.; Li, L.J.; Liaw, D.J. Colorless-to-colorful switching electrochromic polyimides with very high contrast ratio. Nat. Commun. 2019, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Morgan, A.B. The future of flame retardant polymers—Unmet needs and likely new approaches. Polym. Rev. 2019, 59, 25–54. [Google Scholar] [CrossRef]
- Tolando, R.; Zanovello, A.; Ferrara, R.; Iley, J.N.; Manno, M. Inactivation of rat liver cytochrome P450 (P450) by N,N-dimethylformamide and N,N-dimethylacetamide. Toxicol. Lett. 2001, 124, 101–111. [Google Scholar] [CrossRef]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1772. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular firefighting—How modern phosphorus chemistry can help solve the challenge of flame retardancy. Angew. Chem. Int. Ed 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Gall, T.; Knittel, D.; Gutmann, J.S.; Opwis, K. Permanent flame retardant finishing of textiles by allyl-functionalized polyphosphazenes. ACS Appl. Mater. Interfaces 2015, 7, 9349–9363. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Gaan, S.; Malucelli, G. Recent advances for flame retardancy of textiles based on phosphorus chemistry. Polymers 2016, 8, 319. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.K.; Chen, K.J.; Yeh, J.T.; Chen, K.N. Curing and combustion properties of a PU-coating system with UV-reactive phosphazene. J. Appl. Polym. Sci. 2002, 85, 1980–1991. [Google Scholar] [CrossRef]
- Kabisch, B.; Fehrenbacher, U.; Kroke, E. Hexamethoxycyclotriphosphazene as a flame retardant for polyurethane foams. Fire Mater. 2014, 38, 462–473. [Google Scholar] [CrossRef]
- Honarkar, H.; Rahimi, A. Applications of Inorganic Polymeric Materials, III: Polyphosphazenes. Monatsh. Chem. 2007, 138, 923–933. [Google Scholar] [CrossRef]
- Gleria, M.; Bolognesi, A.; Porzio, W.; Catellani, M.; Destri, S.; Audisio, G. Grafting reactions onto poly (organophosphazenes). I. The case of poly [bis(4-isopropylphenoxy) phosphazene-g-polystyrene copolymers. Macromolecules 1987, 20, 469–473. [Google Scholar] [CrossRef]
- Lejeune, N.; Dez, I.; Jaffres, P.A.; Lohier, J.F.; Madec, P.J.; Santos, J.S.O. Synthesis, crystal structure and thermal properties of phosphorylated cyclotriphosphazenes. Eur. J. Inorg. Chem. 2018, 2018, 138–143. [Google Scholar] [CrossRef]
- Zanini, S.; Riccavdi, C.; Orlandi, M.; Colombo, C.; Croccolo, F. Plasma-induced graft-polymerisation of ethylene glycol methacrylate phosphate on polyethylene films. Polym. Degrad. Stab. 2008, 93, 1158–1163. [Google Scholar] [CrossRef]
- Tang, C.; Yan, H.X.; Li, M.N.; Lv, Q. A novel phosphorus-containing polysiloxane for fabricating high performance electronic material with excellent dielectric and thermal properties. J. Mater. Sci. Mater. Electron. 2018, 29, 195–204. [Google Scholar] [CrossRef]
- Qian, L.J.; Ye, L.J.; Xu, G.Z.; Liu, J.; Guo, J.Q. The non-halogen flame retardant epoxy resin based on a novel compound with phosphaphenanthrene and cyclotriphosphazene double functional groups. Polym. Degrad. Stab. 2011, 96, 1118–1124. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, X.; Yu, B.; Feng, X.; Mu, X.; Yuen, R.K.K.; Hu, Y. Flame-retardant-wrapped polyphosphazene nanotubes: A novel strategy for enhancing the flame retardancy and smoke toxicity suppression of epoxy resins. J. Hazard. Mater. 2017, 325, 327–339. [Google Scholar] [CrossRef]
- Yan, Y.W.; Chen, L.; Jian, R.K.; Kong, S.; Wang, Y.Z. Intumescence: An effect way to flame retardance and smoke suppression for polystyrene. Polym. Degrad. Stab. 2012, 97, 1423–1431. [Google Scholar] [CrossRef]















| PI | PPZ (wt.%) | λ (nm) a | T450 (%) b | L* c | a* c | b* c | Haze |
|---|---|---|---|---|---|---|---|
| PI-1 | 0 | 291 | 83.6 | 96.11 | −0.23 | 2.57 | 1.21 |
| PI-2 | 5 | 292 | 81.1 | 95.10 | −0.54 | 4.21 | 3.79 |
| PI-3 | 10 | 294 | 75.0 | 94.87 | −0.27 | 3.56 | 5.03 |
| PI-4 | 15 | 295 | 27.2 | 92.37 | 0.16 | 4.15 | 27.67 |
| PI-5 | 20 | 295 | 9.5 | 87.95 | 0.11 | 3.42 | 93.83 |
| PI-6 | 25 | 298 | 1.1 | 86.98 | 0.09 | 4.65 | 100.00 |
| Samples | PPZ (wt.%) | Tg (°C) a | T10% (°C) b | Tmax (°C) b | Rw700 (%) c |
|---|---|---|---|---|---|
| PPZ | 100 | ND d | 381.3 | 438.2 | 8.2 |
| PI-1 | 0 | 260.6 | 487.3 | 527.7 | 10.0 |
| PI-2 | 5 | 244.8 | 470.2 | 501.7 | 17.1 |
| PI-3 | 10 | 227.4 | 411.6 | 448.9 | 58.3 |
| PI-4 | 15 | 220.9 | 405.5 | 413.2 | 56.3 |
| PI-5 | 20 | 219.8 | 405.1 | 407.1 | 55.2 |
| PI-6 | 25 | 207.3 | 406.6 | 405.0 | 50.8 |
| PI | UL 94 | t1 (s) | t2 (s) | t1 + t2 (s) | Drip** a | Ignition b | LOI c (%) | THR d (MJ/m2) | pHRR e (kW/m2) |
|---|---|---|---|---|---|---|---|---|---|
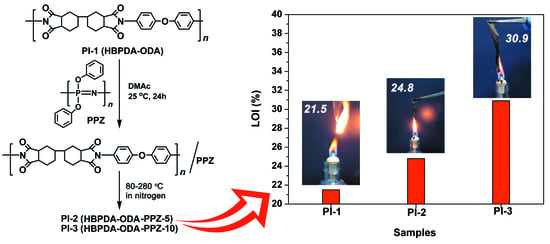
| PI-1 | Not VTM-2 | ND f | ND | ND | Yes | Yes | 21.5 | 1.45 | 98.9 |
| PI-2 | VTM-0 | 11 | 8 | 19 | No | No | 24.8 | 1.48 | 77.3 |
| PI-3 | VTM-0 | 15 | 0 | 15 | No | No | 30.9 | 0.99 | 76.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Jiang, G.; Zhang, Y.; Wu, L.; Jia, Y.; Tan, Y.; Liu, J.; Zhang, X. Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer. Polymers 2020, 12, 90. https://doi.org/10.3390/polym12010090
Wu X, Jiang G, Zhang Y, Wu L, Jia Y, Tan Y, Liu J, Zhang X. Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer. Polymers. 2020; 12(1):90. https://doi.org/10.3390/polym12010090
Chicago/Turabian StyleWu, **ao, Ganglan Jiang, Yan Zhang, Lin Wu, Yanjiang Jia, Yaoyao Tan, **gang Liu, and **umin Zhang. 2020. "Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer" Polymers 12, no. 1: 90. https://doi.org/10.3390/polym12010090