Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Demographic and Clinical Characteristics
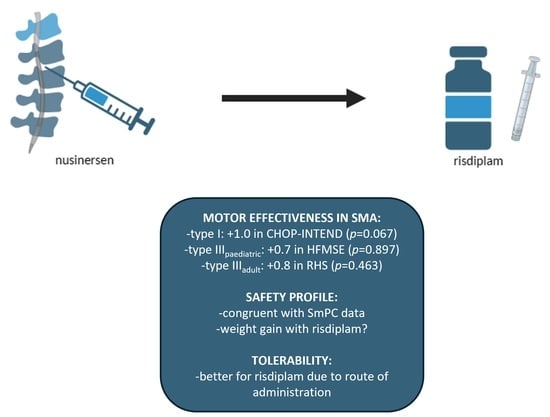
3.2. Effectiveness Outcomes
3.3. Safety Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Darras, B.T. Spinal muscular atrophies. Pediatr. Clin. N. Am. 2015, 62, 743–766. [Google Scholar] [CrossRef]
- Chen, T.H. New and Develo** Therapies in Spinal Muscular Atrophy: From Genotype to Phenotype to Treatment and Where Do We Stand. Int. J. Mol. Sci. 2020, 21, 3297. [Google Scholar] [CrossRef]
- Talbot, K.; Tizzano, E.F. The clinical landscape for SMA in a new therapeutic era. Gene Ther. 2017, 24, 529–533. [Google Scholar] [CrossRef]
- Tizzano, E.F.; Finkel, R.S. Spinal muscular atrophy: A changing phenotype beyond the clinical trials. Neuromuscul. Disord. 2017, 27, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Farrar, M.A.; Carey, K.A.; Paguinto, S.G.; Kasparian, N.A.; De Abreu Lourenço, R. “The Whole Game is Changing and You’ve Got Hope”: Australian Perspectives on Treatment Decision Making in Spinal Muscular Atrophy. Patient 2020, 13, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, J.; Weiss, D.; Schlenker, F.; Groth, M.; Denecke, J. Intrathecal Administration of Nusinersen in Pediatric SMA Patients with and without Spine Deformities: Experiences and Challenges over 3 Years in a Single Center. Neuropediatrics 2021, 52, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Stolte, B.; Totzeck, A.; Kizina, K.; Bolz, S.; Pietruck, L.; Mönninghoff, C.; Guberina, N.; Oldenburg, D.; Forsting, M.; Kleinschnitz, C.; et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418803246. [Google Scholar] [CrossRef] [PubMed]
- Agosto, C.; Benedetti, F.; Salamon, E.; Mercante, A.; Papa, S.; Giacomelli, L.; Santini, A.; Benini, F. How children and caregivers viewed the change from nusinersen to risdiplam for treating spinal muscular atrophy. Acta Paediatr. 2023, 112, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Darras, B.T.; Masson, R.; Mazurkiewicz-Bełdzińska, M.; Rose, K.; **ong, H.; Zanoteli, E.; Baranello, G.; Bruno, C.; Vlodavets, D.; Wang, Y.; et al. Risdiplam-Treated Infants with Type 1 Spinal Muscular Atrophy versus Historical Controls. N. Engl. J. Med. 2021, 385, 427–435. [Google Scholar] [CrossRef]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and efficacy of once-daily risdiplam in type 2 and non-ambulant type 3 spinal muscular atrophy (SUNFISH part 2): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef]
- Paik, J. Risdiplam: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Day, J.W.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Saito, K.; Vuillerot, C.; Baranello, G.; Goemans, N.; Kirschner, J.; et al. Two-year efficacy and safety of risdiplam in patients with type 2 or non-ambulant type 3 spinal muscular atrophy (SMA). J. Neurol. 2023, 270, 2531–2546. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, S.; Boccanegra, B.; Vitturi, G.; Trifirò, G.; De Luca, A. Pharmacological Therapies of Spinal Muscular Atrophy: A Narrative Review of Preclinical, Clinical-Experimental, and Real-World Evidence. Brain Sci. 2023, 13, 1446. [Google Scholar] [CrossRef]
- Belančić, A.; Strbad, T.; Kučan Štiglić, M.; Vitezić, D. Effectiveness of Nusinersen in Type 1, 2 and 3 Spinal Muscular Atrophy: Croatian Real-World Data. J. Clin. Med. 2023, 12, 2839. [Google Scholar] [CrossRef] [PubMed]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. Risdiplam in Patients Previously Treated with Other Therapies for Spinal Muscular Atrophy: An Interim Analysis from the JEWELFISH Study. Neurol. Ther. 2023, 12, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.M.; Arya, K.; Kuntz, N.; Phan, H.C.; Sieburg, C.; Swoboda, K.J.; Veerapandiyan, A.; Assman, B.; Bader-Weder, S.; Dickendesher, T.L.; et al. An expanded access program of risdiplam for patients with Type 1 or 2 spinal muscular atrophy. Ann. Clin. Transl. Neurol. 2022, 9, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.C.; Meiling, J.B.; Cartwright, M.S. A case series evaluating patient perceptions after switching from nusinersen to risdiplam for spinal muscular atrophy. Muscle Nerve 2024, 69, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Ribero, V.A.; Daigl, M.; Martí, Y.; Gorni, K.; Evans, R.; Scott, D.A.; Mahajan, A.; Abrams, K.R.; Hawkins, N. How does risdiplam compare with other treatments for Types 1–3 spinal muscular atrophy: A systematic literature review and indirect treatment comparison. J. Comp. Eff. Res. 2022, 11, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Poirier, A.; Weetall, M.; Heinig, K.; Bucheli, F.; Schoenlein, K.; Alsenz, J.; Bassett, S.; Ullah, M.; Senn, C.; Ratni, H.; et al. Risdiplam distributes and increases SMN protein in both the central nervous system and peripheral organs. Pharmacol. Res. Perspect. 2018, 6, e00447. [Google Scholar] [CrossRef] [PubMed]



| Baseline Demographics | SMA 1 | SMA 3p | SMA 3a | SMA 3 Total | Total | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (% out of total) | 6 (35.3%) | 4 (23.5%) | 7 (41.2%) | 11 (64.7%) | 17 (100%) | ||||
| Age (years) | X ± SD | 8.0 ± 3.6 | 11.4 ± 4.3 | 34.5 ± 8.1 | 26.1 ± 13.4 | 19.7 ± 14.0 | |||
| Med (Min–Max) | 8.0 (3.0–12.75) | 10.1 (7.75–17.5) | 35.0 (21.25–44.5) | 28.5 (7.75–44.5) | 12.75 (3.0–44.5) | ||||
| Male/female | 3/3 | 0/4 | 4/3 | 4/7 | 7/10 | ||||
| SMN1 exon 7 copy number | 0 | 6 | 4 | 7 | 11 | 17 | |||
| 1 | 0 | 0 | 0 | 0 | 0 | ||||
| SMN1 exon 8 copy number | 0 | 6 | 3 | 4 | 7 | 13 | |||
| 1 | 0 | 1 | 2 | 3 | 3 | ||||
| 2 | 0 | 0 | 1 | 1 | 1 | ||||
| SMN2 exon 7 copy number | 1 | 0 | 0 | 0 | 0 | 0 | |||
| 2 | 5 | 1 | 0 | 1 | 6 | ||||
| 3 | 1 | 3 | 4 | 7 | 8 | ||||
| 4 | 0 | 0 | 3 | 3 | 3 | ||||
| SMN2 exon 8 copy number | 1 | 0 | 0 | 0 | 0 | 0 | |||
| 2 | 5 | 0 | 2 | 2 | 7 | ||||
| 3 | 1 | 3 | 4 | 7 | 8 | ||||
| 4 | 0 | 1 | 1 | 2 | 2 | ||||
| NAIP copy number | 0 | 4 | 1 | 0 | 1 | 5 | |||
| 1 | 1 | 0 | 2 | 2 | 3 | ||||
| 2 | 1 | 3 | 4 | 7 | 8 | ||||
| 3 | 0 | 0 | 1 | 1 | 1 | ||||
| Mechanical ventilation—N (% out of total for corresponding SMA type) | 3 (50.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (17.6%) | ||||
| BiPAP—N (% out of total for corresponding SMA type) | 3 (50.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 3 (17.6%) | ||||
| PEG—N (% out of total for corresponding SMA type) | 2 (33.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11.8%) | ||||
| NG tube—N (% out of total for corresponding SMA type) | 2 (33.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (11.8%) | ||||
| Baseline motor function (N of patients) | X ± SD | CHOP INTEND | 33.7 ± 23.7 | HFMSE | 54.8 ± 10.2 | RHS | 39.1 ± 12.7 | N/A | N/A |
| Med (Min–Max) | 31.5 (9–58) | 58.5 (40–62) | 37 (19–57) | ||||||
| Time of Evaluation | N of Patients | Motoric Score/X ± SD | Change from Baseline | p Value | ||||
|---|---|---|---|---|---|---|---|---|
| SMA 1 | ||||||||
| Baseline | 6 | 33.7 ± 23.7 | N/A | 0.067 | ||||
| 6 months after switch | 6 | 34.3 ± 23.6 | 0.6 | |||||
| 12 months after switch | 6 | 34.7 ± 23.3 | 1.0 | |||||
| SMA 3p | ||||||||
| Baseline | 4 | 54.8 ± 10.2 | N/A | 0.897 | ||||
| 6 months after switch | 4 | 55.0 ± 10.2 | 0.2 | |||||
| 12 months after switch | 4 | 55.5 ± 11.3 | 0.7 | |||||
| SMA 3a | ||||||||
| Baseline | 7 * | 6 | 39.1 ± 12.7 * | 39.5±13.9 | N/A * | N/A | N/A * | 0.463 |
| 6 months after switch | 40.0 ± 13.1 * | 40.5±14.3 | 0.9 * | 1.0 | 0.141 * | |||
| 12 months after switch | N/A * | 40.3 ± 15.6 | N/A * | 0.8 | N/A * | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belančić, A.; Strbad, T.; Kučan Štiglić, M.; Vitezić, D. Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety. J. Pers. Med. 2024, 14, 244. https://doi.org/10.3390/jpm14030244
Belančić A, Strbad T, Kučan Štiglić M, Vitezić D. Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety. Journal of Personalized Medicine. 2024; 14(3):244. https://doi.org/10.3390/jpm14030244
Chicago/Turabian StyleBelančić, Andrej, Tea Strbad, Marta Kučan Štiglić, and Dinko Vitezić. 2024. "Switching from Nusinersen to Risdiplam: A Croatian Real-World Experience on Effectiveness and Safety" Journal of Personalized Medicine 14, no. 3: 244. https://doi.org/10.3390/jpm14030244