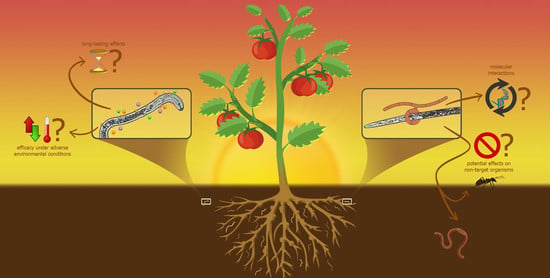
The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents
Abstract
:1. Introduction
2.1. Root-Knot Nematodes (RKNs), Meloidogyne spp.
2.2. Cyst Nematodes (CNs), Globodera, and Heterodera spp.
2.3. Root-Lesion Nematodes (RLNs), Pratylenchus spp.
2.4. Pinewood Nematode (PWN), Bursaphelenchus xylophilus
2.5. Reniform Nematode (RN), Rotylenchulus reniformis
2.6. Fanleaf Virus Nematode (FVN), **phinema index
2.7. Other Economically Important PPNs
3. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Bardgett, R.D.; van der Putten, W. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, S. The Phylogenetic Systematics of Freeliving Nematodes; Platt, H.M., Ed.; Ray Society: London, UK, 1994. [Google Scholar]
- Ferris, H. Contribution of nematodes to the structure and function of the soil food web. J. Nematol. 2010, 42, 63–67. [Google Scholar] [PubMed]
- Ferris, H.; Venette, R.; Scow, K. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl. Soil Ecol. 2004, 25, 19–35. [Google Scholar] [CrossRef]
- Elling, A.A. Major Emerging Problems with Minor Meloidogyne Species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- EU Commission Implementing Regulation (EU) 2019/2072. Available online: https://eur-lex.europa.eu/eli/reg_impl/2019/2072/2021-12-16 (accessed on 31 August 2022).
- EPPO A2 List. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list#nematodes (accessed on 31 August 2022).
- Categorization for Asia and Pacific Plant Protection Commission. Available online: https://gd.eppo.int/rppo/APPPC/categorization (accessed on 6 October 2022).
- Categorization for Inter-African Phytosanitary Council. Available online: https://gd.eppo.int/rppo/IAPSC/categorization (accessed on 6 October 2022).
- Animal and Plant Health Inspection Service (APHIS). Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/import-information/rppl/rppl-table (accessed on 31 August 2022).
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [Green Version]
- Kantor, M.; Handoo, Z.; Kantor, C.; Carta, L. Top Ten Most Important U.S.-Regulated and Emerging Plant-Parasitic Nematodes. Horticulturae 2022, 8, 208. [Google Scholar] [CrossRef]
- Mamiya, Y. Pathology of the Pine Wilt Disease Caused by Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 1983, 21, 201–220. [Google Scholar] [CrossRef]
- Chitwood, D.J. Nematicides. In Encyclopedia of Agrochemicals; Plimmer, J.R., Ed.; John Wiley & Sons: New York, NY, USA, 2003; Volume 3, pp. 1104–1115. [Google Scholar]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St. Paul, MN, USA, 1983; ISBN 978-0-89054-053-4. [Google Scholar]
- Mesa-Valle, C.M.; Garrido-Cardenas, J.A.; Cebrian-Carmona, J.; Talavera, M.; Manzano-Agugliaro, F. Global Research on Plant Nematodes. Agronomy 2020, 10, 1148. [Google Scholar] [CrossRef]
- Topalović, O.; Hussain, M.; Heuer, H. Plants and Associated Soil Microbiota Cooperatively Suppress Plant-Parasitic Nematodes. Front. Microbiol. 2020, 11, 313. [Google Scholar] [CrossRef] [Green Version]
- Stirling, G.R. Biological Control of Plant-Parasitic Nematodes: An Ecological Perspective, a Review of Progress and Opportunities for Further Research. In Biological Control of Plant-Parasitic Nematodes: Building Coherence Between Microbial Ecology and Molecular Mechanisms; Davies, K.G., Spiegel, Y., Eds.; Springer: Berlin, Germany, 2011; pp. 1–38. [Google Scholar]
- Baker, K.; Cook, R.J. Biological Control of Plant Pathogens; W.H. Freeman and Company: San Francisco, CA, USA, 1974; ISBN 9780716705895. [Google Scholar]
- Viaene, N.; Coyne, D.L.; Davies, K.G. Biological and Cultural Management. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 383–410. [Google Scholar]
- Al-Ani, L.K.T.; Soares, F.E.D.F.; Sharma, A.; Santos-Villalobos, S.D.L.; Valdivia-Padilla, A.V.; Aguilar-Marcelino, L. Strategy of Nematophagous Fungi in Determining the Activity of Plant Parasitic Nematodes and Their Prospective Role in Sustainable Agriculture. Front. Fungal Biol. 2022, 3, 1–9. [Google Scholar] [CrossRef]
- Jiang, X.; ** Fungi. Microbiol. Spectr. 2017, 5, 963–974. [Google Scholar] [CrossRef]
- Noura, C.H.; Hajer, R.; Asma, L.; Lobna, H.H.; Najet, H.-R. Antagonistic Potential of Verticillium leptobactrum against Pratylenchus vulnus Associated with Apple Rootstock. J. Entomol. Zool. Stud. 2018, 6, 172–176. [Google Scholar]
- Sankaranarayanan, C.; Hari, K. Integration of Arbuscular Mycorrhizal and Nematode Antagonistic Fungi for the Biocontrol of Root Lesion Nematode Pratylenchus zeae Graham, 1951 on Sugarcane. Sugar Tech 2020, 23, 194–200. [Google Scholar] [CrossRef]
- McDonald, M.R.; Ives, L.; Adusei-Fosu, K.; Jordan, K.S. Ditylenchus dipsaci and Fusarium oxysporum on garlic: One plus one does not equal two. Can. J. Plant Pathol. 2021, 43, 749–759. [Google Scholar] [CrossRef]
- Wang, C.Y.; Yin, C.; Fang, Z.M.; Wang, Z.; Wang, Y.B.; Xue, J.J.; Gu, L.J.; Sung, C.K. Using the nematophagous fungus Esteya vermicola to control the disastrous pine wilt disease. Biocontrol Sci. Technol. 2018, 28, 268–277. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Yin, C.; Gao, J.; Tao, R.; Sun, Y.; Wang, C.; Wang, Z.; Li, Y.; Sung, C. In vivo infection of Bursaphelenchus xylophilus by the fungus Esteya vermicola. Pest Manag. Sci. 2020, 76, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.X.; Liu, Z.K.; Wen, X.J.; Zi, Z.S.; Feng, Y.Q.; Zhang, W.; Li, D.Z.; Zhang, X.Y. Isolation and identification of Esteya vermicola and its potential for controlling pinewood nematode. For. Pathol. 2022, 52, e12745. [Google Scholar] [CrossRef]
- Yin, C.; Wang, Y.; Zhang, Y.A.; Wang, H.; Duan, B.; Tao, R.; Gao, J.; Sung, C. keun Hypothesized Mechanism of Biocontrol against Pine Wilt Disease by the Nematophagous Fungus Esteya vermicola. Eur. J. Plant Pathol. 2020, 156, 811–818. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Araújo, J.P.M.; Zhang, X.; Ji, Y.; Hulcr, J. Esteya floridanum sp. nov.: An Ophiostomatalean Nematophagous Fungus and Its Potential to Control the Pine Wood Nematode. Phytopathology 2021, 111, 304–311. [Google Scholar] [CrossRef]
- Vicente, C.S.L.; Soares, M.; Faria, J.M.S.; Espada, M.; Mota, M.; Nóbrega, F.; Ramos, A.P.; Inácio, M.L. Fungal Communities of the Pine Wilt Disease Complex: Studying the Interaction of Ophiostomatales With Bursaphelenchus xylophilus. Front. Plant Sci. 2022, 13, 1650. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Jiang, Z.; Bai, Q.; Wu, S.; Wang, Y.; Li, C.; Zeng, X.; Gan, X.; **e, X.; et al. A new strain of Volutella citrinella with nematode predation and nematicidal activity, isolated from the cysts of potato cyst nematodes in China. BMC Microbiol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Lira, V.L.; Santos, D.V.; Barbosa, R.N.; Costa, A.F.; Maia, L.C.; Moura, R.M. Biocontrol Potential of Fungal Filtrates on the Reniform Nematode (Rotylenchulus reniformis) in Coriander and Cowpea. Nematropica 2020, 50, 86–95. [Google Scholar]
- Bernardo, V.F.; Garita, S.A.; Arango, M.C.; Ripodas, J.I.; Saparrat, M.C.N.; Ruscitti, M.F. Arbuscular mycorrhizal fungi against the false root-knot nematode activity in Capsicum annuum: Physiological responses in plants. Biocontrol Sci. Technol. 2020, 31, 119–131. [Google Scholar] [CrossRef]
- Subbotin, S.A.; Palomares-Rius, J.E.; Castillo, P. Systematics of Root-Knot Nematodes (Nematoda: Meloidogynidae); Subbotin, S.A., Palomares-Rius, J.E., Castillo, P., Eds.; Brill: Boston, MA, USA, 2021; Volume 14, ISBN 978-90-04-38758-4. [Google Scholar]
- Hockland, S.; Niere, B.; Grenier, E.; Blok, V.; Phillips, M.; Nijs, L.D.; Anthoine, G.; Pickup, J.; Viaene, N. An evaluation of the implications of virulence in non-European populations of Globodera pallida and G. rostochiensis for potato cultivation in Europe. Nematology 2012, 14, 1–13. [Google Scholar] [CrossRef]
- Grainger, J. Factors Affecting the Control of Eelworm Diseases. Nematologica 1964, 10, 5–20. [Google Scholar] [CrossRef]
- Turner, S.J. Population decline of potato cyst nematodes (Globodera rostochiensis, G. pallida) in field soils in Northern Ireland. Ann. Appl. Biol. 1996, 129, 315–322. [Google Scholar] [CrossRef]
- Turner, S.J.; Subbotin, S.A. Cyst Nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 109–143. [Google Scholar]
- Tylka, G.L.; Marett, C.C. Known Distribution of the Soybean Cyst Nematode, Heterodera glycines, in the United States and Canada in 2020. Plant Health Prog. 2021, 22, 72–74. [Google Scholar] [CrossRef]
- The Soybean Cyst Nematode. Available online: https://conservancy.umn.edu/handle/11299/94033 (accessed on 7 October 2022).
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; den Nijs, L.; Hockland, S.; Maafi, Z.T. Current Nematode Threats to World Agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J.T., Gheysen, G., Fenoll, C., Eds.; Springer: Heidelberg, Germany, 2011; pp. 21–43. [Google Scholar]
- Kerry, B.; Irving, F. Variation Between Strains of the Nematophagous Fungus, Verticillium chlamydosporium Goddard. Ii. Factors Affecting Parasitism of Cyst Nematode Eggs. Nematologica 1986, 32, 474–485. [Google Scholar] [CrossRef]
- Castillo, P.; Vovlas, N. Pratylenchus (Nematoda: Pratylenchidae): Diagnosis, Biology, Pathogenecity and Management; E. J. Brill: Leiden, The Netherlands, 2007. [Google Scholar]
- Jones, M.; Fosu-Nyarko, J. Molecular biology of root lesion nematodes (Pratylenchus spp.) and their interaction with host plants. Ann. Appl. Biol. 2014, 164, 163–181. [Google Scholar] [CrossRef]
- Bélair, G.; Coulombe, J.; Dauphinais, N. Management of Pratylenchus penetrans and Verticilllium symptoms in strawberry. Phytoprotection 2018, 98, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Janssen, T.; Karssen, G.; Orlando, V.; Subbotin, S.A.; Bert, W. Molecular characterization and species delimiting of plant-parasitic nematodes of the genus Pratylenchus from the penetrans group (Nematoda: Pratylenchidae). Mol. Phylogenetics Evol. 2017, 117, 30–48. [Google Scholar] [CrossRef]
- Handoo, Z.; Yan, G.; Kantor, M.; Huang, D.; Chowdhury, I.; Plaisance, A.; Bauchan, G.; Mowery, J. Morphological and Molecular Characterization of Pratylenchus dakotaensis n. sp. (Nematoda: Pratylenchidae), a New Root-Lesion Nematode Species on Soybean in North Dakota, USA. Plants 2021, 10, 168. [Google Scholar] [CrossRef]
- Tares, S. Use of Species-Specific Satellite DNA from Bursaphelenchus xylophilus as a Diagnostic Probe. Phytopathology 1994, 84, 294–298. [Google Scholar] [CrossRef]
- Tokushige, Y.; Kiyohara, T. Bursaphelenchus Sp. in the Wood of Dead Pine Trees. J. Jpn. For. Soc. 1969, 51, 193–195. [Google Scholar] [CrossRef]
- Vicente, C.S.L.; Soares, M.; Faria, J.M.S.; Ramos, A.P.; Inácio, M.L. Insights into the Role of Fungi in Pine Wilt Disease. J. Fungi 2021, 7, 780. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.; Cardoso, J.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The pinewood nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Liou, J.; Shih, J.; Tzean, S. Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol. Res. 1999, 103, 242–248. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Inácio, M.L.; Mota, M. The Potential of Esteya spp. for the Biocontrol of the Pinewood Nematode, Bursaphelenchus xylophilus. Microorganisms 2022, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Rabiey, M.; Hailey, L.E.; Roy, S.R.; Grenz, K.; Al-Zadjali, M.A.S.; Barrett, G.A.; Jackson, R.W. Endophytes vs Tree Pathogens and Pests: Can They Be Used as Biological Control Agents to Improve Tree Health? Eur. J. Plant Pathol. 2019, 155, 711–729. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, K.S. Reniform nematode (Rotylenchulus reniformis) and its interactions with cotton (Gossypium hirsutum). In Integrated Nematode Management: State-of-the-Art and Visions for the Future; Sikora, R.A., Desaeger, J., Molendijk, L.P.G., Eds.; CAB International: Wallingford, UK, 2021; pp. 94–99. [Google Scholar] [CrossRef]
- McSorley, R. Nematodes Associated with Sweetpotato and Edible Aroids in Southern Florida. Proc. Fla. State Hortic. Soc. 1980, 93, 283–285. [Google Scholar]
- McSorley, R.; Pohronezny, K.; Stall, W.M. Aspects of Nematode Control on Snap Bean with Emphasis on the Relationship between Nematode Density and Plant Damage. Proc. Fla. State Hortic. Soc. 1981, 94, 134–136. [Google Scholar]
- McSorley, R.; Campbell, C.W.; Parrado, J.L. Nematodes Associated with Tropical and Subtropical Fruit Trees in South Florida. Proc. Fla. State Hortic. Soc. 1982, 95, 132–135. [Google Scholar]
- Robinson, A.F.; Inserra, R.N.; Caswell-Chen, E.P.; Vovlas, N.; Troccoli, A. Review: Rotylenchulus Species: Identification, Distribution, Host Ranges, and Crop Plant Resistance. Nematropica 1997, 27, 127–180. [Google Scholar]
- Wang, K.H.; Hooks, C.R.R. Plant-Parasitic Nematodes and Their Associated Natural Enemies within Banana (Musa spp.) Plantings in Hawaii. Nematropica 2009, 39, 57–73. [Google Scholar]
- Nguyen, K.; Rosso, L.; Gozel, U.; Duncan, L.; Adams, B.; Agostinelli, A.; Lamberti, F. Molecular and morphological consilience in the characterisation and delimitation of five nematode species from Florida belonging to the **phinema americanum-group. Nematology 2006, 8, 521–532. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.E.; Brown, D.J.F. Nematode Vectors of Plant Viruses; CAB International: Wallingford, UK, 1997. [Google Scholar]
- Van Zyl, S.; Vivier, M.; Walker, M. **phinema index and its Relationship to Grapevines: A review. South Afr. J. Enol. Vitic. 2016, 33, 21–32. [Google Scholar] [CrossRef] [Green Version]
- Jelly, N.S.; Schellenbaum, P.; Walter, B.; Maillot, P. Transient expression of artificial microRNAs targeting Grapevine fanleaf virus and evidence for RNA silencing in grapevine somatic embryos. Transgenic Res. 2012, 21, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Coomans, A.; Huys, R.; Heyns, J.; Luc, M. Character Analysis, Phylogeny and Biogeography of the Genus **phinema Cobb, 1913 (Nematoda: Longidoridae). Ann. Musée R. De L’afrique Cent. Tervuren 2001, 287, 1–239. [Google Scholar] [CrossRef]
- Groza, M.; Lazarova, S.; Costache, C.; De Luca, F.; Rosca, I.; Fanelli, E.; Peneva, V. Morphological characterisation and diagnostics of **phinema non-americanum group species (Nematoda: Longidoridae) from Romania using mutiplex PCR. Helminthologia 2013, 50, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gutiérrez, C.; Bravo, M.A.; Santos, M.T.; Vieira, P.; Mota, M. An update on the genus Longidorus, Paralongidorus and **phinema (Family Longidoridae) in Portugal. Zootaxa 2016, 4189, 99–114. [Google Scholar] [CrossRef]
- Nicol, J.; Stirling, G.; Rose, B.; May, P.; VAN Heeswijck, R. Impact of nematodes on grapevine growth and productivity: Current knowledge and future directions, with special reference to Australian viticulture. Aust. J. Grape Wine Res. 1999, 5, 109–127. [Google Scholar] [CrossRef]
- Orton Williams, K.J.; Siddiqi, M.R. Radopholus similis. In C.I.H. Descriptions of Plant-Parasitic Nematodes; Willmott, S., Gooch, P.S., Siddiqi, M.R., Franklin, M., Eds.; Commonnwealth Institute of Helminthology: Hertfordshire, UK, 1973; Volume 2, p. 4. [Google Scholar]
- Duncan, L.W.; Moens, M. Migratory endoparasitic nematodes. In Plant Nematology; Perry, R.N., Moens, M., Eds.; CAB International: Wallingford, UK, 2013; pp. 144–178. [Google Scholar] [CrossRef]
- Franklin, M.T.; Siddiqi, M.R. Aphelenchoides besseyi. In C.I.H. Descriptions of Plant-Parasitic Nematodes; Willmott, S.M., Gooch, P.S., Siddiqi, M.R., Franklin, M.T., Eds.; Commonnwealth Institute of Helminthology: Hertfordshire, UK, 1972; Volume 1. [Google Scholar]
- Bridge, J.; Plowright, R.A.; Deliang, P. Nematode Parasites of Rice. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture; Luc, M., Sikora, R.A., Bridge, J., Eds.; CAB International: Wallingford, UK, 2005; pp. 87–130. [Google Scholar]
- Rybarczyk-Mydłowska, K.; Mooyman, P.; van Megen, H.; Elsen, S.V.D.; Vervoort, M.; Veenhuizen, P.; van Doorn, J.; Dees, R.; Karssen, G.; Bakker, J.; et al. Small Subunit Ribosomal DNA-Based Phylogenetic Analysis of Foliar Nematodes (Aphelenchoides spp.) and Their Quantitative Detection in Complex DNA Backgrounds. Phytopathology 2012, 102, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Ehlers, R.-U. Cost-Benefit, Risk and Trade-off Analysis of Regulation. In Regulation of Biological Control Agents; Ehlers, R.-U., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 139–153. ISBN 9789048136636. [Google Scholar]
- Subedi, P.; Gattoni, K.; Liu, W.; Lawrence, K.S.; Park, S.-W. Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes. Plants 2020, 9, 1167. [Google Scholar] [CrossRef] [PubMed]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2017, 63, 39–59. [Google Scholar] [CrossRef] [Green Version]
- Mei, X.; Wang, X.; Li, G. Pathogenicity and Volatile Nematicidal Metabolites from Duddingtonia flagrans against Meloidogyne incognita. Microorganisms 2021, 9, 2268. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Yan, W.; Wei, S.; Wang, Z.; Zhao, S.; Cao, L.; Rajput, N.A.; Ye, Y. Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiol. Lett. 2019, 366, fnz169. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.-X.; Li, G.-H.; Li, C.-Q.; Wang, S.-L.; Pan, X.-R.; Zhang, K.-Q.; Zhao, P.-J. Identification of Nematicidal Metabolites from Purpureocillium lavendulum. Microorganisms 2022, 10, 1343. [Google Scholar] [CrossRef] [PubMed]
- Abebew, D.; Sayedain, F.S.; Bode, E.; Bode, H.B. Uncovering Nematicidal Natural Products from Xenorhabdus Bacteria. J. Agric. Food Chem. 2022, 70, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Rupcic, Z.; Chepkirui, C.; Hernández-Restrepo, M.; Crous, P.; Luangsa-Ard, J.J.; Stadler, M. New nematicidal and antimicrobial secondary metabolites from a new species in the new genus, Pseudobambusicola thailandica. MycoKeys 2018, 33, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.J. An Alternative Perspective for the Theory of Biological Control. Insects 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sentis, A.; Hemptinne, J.; Margo, A.; Outreman, Y. Biological control needs evolutionary perspectives of ecological interactions. Evol. Appl. 2022. [Google Scholar] [CrossRef]
- Szűcs, M.; Vercken, E.; Bitume, E.V.; Hufbauer, R.A. The implications of rapid eco-evolutionary processes for biological control—A review. Èntomol. Exp. Appl. 2019, 167, 598–615. [Google Scholar] [CrossRef] [Green Version]
- Aguilar-Fenollosa, E.; A Jacas, J. Can we forecast the effects of climate change on entomophagous biological control agents? Pest Manag. Sci. 2013, 70, 853–859. [Google Scholar] [CrossRef]
- Castex, V.; Beniston, M.; Calanca, P.; Fleury, D.; Moreau, J. Pest management under climate change: The importance of understanding tritrophic relations. Sci. Total Environ. 2018, 616–617, 397–407. [Google Scholar] [CrossRef]
- Tougeron, K.; Tena, A. Hyperparasitoids as new targets in biological control in a global change context. Biol. Control 2018, 130, 164–171. [Google Scholar] [CrossRef]


| Major PPN | Biocontrol Agent(s) | Nematode Species | Plant Host | Reference |
|---|---|---|---|---|
| Root-knot nematodes (Meloidogyne spp.) | Bacillus subtilis | Meloidogyne spp. | Sugarcane | [41] |
| B. cereus, B. subtilis, B. thuringiensis, Priestia megaterium (basionym: B. megaterium) | Soybean | [42] | ||
| Pasteuria penetrans | M. arenaria | Peanut | [43] | |
| Pseudomonas putida + Trichoderma harzianum | M. graminicola | Rice | [44] | |
| Bacillus sp., Paenibacillus sp., Xanthomonas sp. | [45] | |||
| Brevundimonas sp., Microbacterium spp. | M. hapla | – | [46] | |
| Cytobacillus firmus (basionym: Bacillus firmus) | M. incognita | Cucumber and tomato | [31] | |
| Bacillus velezensis | Cucumber | [32] | ||
| Bacillus thuringiensis, B. velezensis | Tomato | [47] | ||
| Bacillus cereus, B. halotolerans, Cytobacilluskochii (basionym: B. kochii), Cytobacillus oceanisediminis (basionym: B. oceanisediminis), B. pseudomycoides, B. pumilus, B. toyonensis, Pseudomonas aeruginosa | [48] | |||
| Brucella pseudogrignonensis (basionym: Ochrobactrum pseudogrignonense) | [49] | |||
| Bacillus velezensis | Cucumber | [50] | ||
| Streptomyces antibioticus | Tomato | [51] | ||
| Paenibacillus alvei, Priestia aryabhattai (basionym: Bacillus aryabhattai) | Tomato and carrot | [52] | ||
| Burkholderia arboris | Tobacco | [53] | ||
| Agrobacterium radiobacter, Bacillus subtilis, Streptomyces spp. | Tomato | [54] | ||
| Bacillus cereus, B. licheniformis, Lysinibacillus sphaericus, P. brassicacearum, P. fluorescens | [55] | |||
| Serratia proteamaculans | [56] | |||
| Bacillus cereus, Pseudomonas putida | Patchouli | [57] | ||
| Pasteuria penetrans | Tomato | [58] | ||
| Bacillus safensis, Lysinibacillus fusiformis, Priestia megaterium (basionym: B. megaterium), Pseudomonas resinovorans, Sphingobacterium daejeonense | M. javanica | Tomato | [59] | |
| Bacillus halotolerans | [60] | |||
| Pseudomonas fluorescens | Tomato and cucumber | [61] | ||
| Bacillus altitudinis | Eggplant and cucumber | [62] | ||
| Bacillus sp., Pseudomonas sp. | Garlic and soybean | [63] | ||
| Pasteuria penetrans | Sugarcane | [64] | ||
| Olive | [65] | |||
| Tomato | [58] | |||
| Cyst nematodes (Globodera and Heterodera spp.) | Bacillus cereus, B. pumilus, B. subtilis, Priestia flexa (basionym: B. flexus), P. megaterium (basionym: B. megaterium) | G. rostochiensis | Potato | [66] |
| Bacillus spp. | H. avenae | Wheat | [67] | |
| Bacillus cereus, B. mycoides (basionym: B. weihenstephanensis), B. thuringiensis | [68] | |||
| Priestia aryabhattai (basionym: Bacillus aryabhattai) | H. glycines | Soybean | [69] | |
| Pasteuria nishizawae | [70] | |||
| Ensifer fredii (basionym: Sinorhizobium fredii) | [71] | |||
| Cytobacillus firmus (basionym: Bacillus firmus) | H. schachtii | Arabidopsis thaliana | [30] | |
| Root lesion nematodes (Pratylenchus spp.) | Bacillus subtilis | Pratylenchus spp. | Sugarcane | [41] |
| Bacillus spp., Pseudomonas sp. | P. coffeae | Coffee | [72] | |
| Bacillus cereus sensu lato, B. mycoides | – | [73] | ||
| Streptomyces microflavus (basionym: Streptomyces fulvissimus), S. venezuelae, S. anulatus, Pseudomonas donghuensis, Pseudomonas sp. | P. penetrans | Onion | [74] | |
| Burrowing nematode (Radopholus similis) | Pseudomonas fluorescens + Purpureocillium lilacinum | R. similis | Banana | [75] |
| Bacillus subtilis + Purpureocillium lilacinum | ||||
| Stem and bulb nematode (Ditylenchus dipsaci) | Bacillus sp., Pseudomonas sp. | Ditylenchus spp. | Garlic | [63] |
| Pinewood nematode (Bursaphelenchus xylophilus) | Escherichia coli, Serratia sp. | B. xylophilus | – | [76] |
| Reniform nematode (Rotylenchulus reniformis) | Bacillus mojavensis, B. velezensis | R. reniformis | Soybean | [77] |
| Fanleaf virus nematode (**phinema index) | Bacillus amyloliquefaciens, B. mycoides (basionym: B. weihenstephanensis, B. thuringiensis, Peribacillus frigoritolerans (basionym: Brevibacterium frigoritolerans, Priestia megaterium (basionym: B. megaterium), Pseudomonas fluorescens | X. index | Grapevine | [78] |
| Fake root-knot nematode (Nacobbus aberrans) | Serratia sp. | N. aberrans | – | [79] |
| Serratia ureilytica | Chili pepper | [80] | ||
|
White tip nematode (Aphelenchoides besseyi) | Xenorhabdus bovienii | A. besseyi | Rice | [81] |
| Bacillus thuringiensis |
| Major PPN | Biocontrol Agent(s) | Nematode Species | Plant Host | Reference |
|---|---|---|---|---|
| Root-knot nematodes (Meloidogyne spp.) | Trichoderma asperellum | Meloidogyne spp. | Tomato | [82] |
| Trichoderma viride | M. graminicola | Rice | [83] | |
| Purpureocillium lilacinum, Trichoderma viride | M. incognita | Cucumber | [84] | |
| Trichoderma asperellum, T. harzianum | Cucumber and tomato | [36] | ||
| Lecanicillium muscarium | Tomato | [85] | ||
| Trichoderma harzianum | [37] | |||
| Penicillium chrysogenum | – | [86] | ||
| Pochonia chlamydosporia | Tomato and cucumber | [34] | ||
| Pochonia chlamydosporia | Tomato | [87] | ||
| Pochonia chlamydosporia | Chickpea | [38] | ||
| Arthrobotrys oligospora, Glomus faciculatum, Purpureocillium lilacinum | Cucumber | [88] | ||
| Glomus spp., G. mosseae, G. viscosum, Pochonia chlamydosporia, Trichoderma harzianum | Tomato | [54] | ||
| Metarhizium anisopliae | [89] | |||
| Purpureocillium lilacinum | M. incognita and M. javanica | [90] | ||
| Arthrobotrys brochopaga, A. oligospora, Monacrosporium thaumasium, Purpureocillium lilacinum, Talaromyces assiutensis, Trichoderma asperellum, T. hamatum, T. harzianum | M. javanica | Tomato | [91] | |
| Pycnoporus sanguineus | [92] | |||
| Cyst nematodes (Globodera and Heterodera spp.) | Pochonia chlamydosporia | G. pallida | Potato | [87] |
| Beauveria bassiana | H. filipjevi | Wheat | [93] | |
| Glomus etunicatum | H. glycines | Soybean | [94] | |
| Root lesion nematodes (Pratylenchus spp.) | Trichoderma spp. | P. brachyurus | Soybean | [95] |
| Pochonia chlamydosporia | Soybean and corn | [96] | ||
| Purpureocillium lilacinum, Trichoderma harzianum | Soybean | [97] | ||
| Trichoderma asperellum | [98] | |||
| Acaulospora longula, Claroideoglomus claroideum, Glomus intraradices and other unidentified AMF | P. penetrans | Apple | [99] | |
| Clonostachys rosea | Wheat | [100] | ||
| Verticillium leptobactrum | P. vulnus | Apple | [101] | |
| Arthrobotrys oligospora, Glomus fasciculatum | P. zeae | Sugarcane | [102] | |
| Burrowing nematode (Radopholus similis) | Purpureocillium lilacinum + Pseudomonas fluorescens | R. similis | Banana [1] | |
| Purpureocillium lilacinum + Bacillus subtilis | ||||
| Stem and bulb nematode (Ditylenchus dipsaci) | Fusarium oxysporum f. sp. cepae | D. dipsaci | Garlic | [103] |
| Pinewood nematode (Bursaphelenchus xylophilus) | Esteya vermicola | B. xylophilus | Pinus densiflora | [104,105,106,107] |
| Esteya floridanum | Pinus koraiensis and Larix olgensis | [108] | ||
| Leptographium spp., Leptographium terebrantis, Graphilbum spp., Ophiostoma ips | – | [109] | ||
| Volutella citrinella | – | [110] | ||
| Reniform nematode (Rotylenchulus reniformis) | Fusarium inflexum, Thielavia terricola, Trichoderma brevicompactum, T. harzianum, T. longibrachiatum, Penicillium citrinum | R. reniformis | Coriander and cowpea | [111] |
| Fake root-knot nematode (Nacobbus aberrans) | Rhizophagus intraradices | N. aberrans | Chili pepper | [112] |
|
White tip nematode (Aphelenchoides besseyi) | Purpureocillium lilacinum | A. besseyi | Rice | [81] |
| Volutella citrinella | – | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens 2022, 11, 1178. https://doi.org/10.3390/pathogens11101178
Pires D, Vicente CSL, Menéndez E, Faria JMS, Rusinque L, Camacho MJ, Inácio ML. The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents. Pathogens. 2022; 11(10):1178. https://doi.org/10.3390/pathogens11101178
Chicago/Turabian StylePires, David, Cláudia S. L. Vicente, Esther Menéndez, Jorge M. S. Faria, Leidy Rusinque, Maria J. Camacho, and Maria L. Inácio. 2022. "The Fight against Plant-Parasitic Nematodes: Current Status of Bacterial and Fungal Biocontrol Agents" Pathogens 11, no. 10: 1178. https://doi.org/10.3390/pathogens11101178