Evaluation of Copper-Contaminated Marginal Land for the Cultivation of Vetiver Grass (Chrysopogon zizanioides) as a Lignocellulosic Feedstock and its Impact on Downstream Bioethanol Production
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Characterization
2.2. Greenhouse Study
2.3. Optimization of Fermentation Parameters
2.4. Effects of Copper Exposure on Lignocellulosic Biomass
2.5. Statistical Analysis
3. Results and Discussion
3.1. Growth, Lignocellulosic Composition and Metal Uptake by Vetiver Grass Grown in Stamp Sand
3.2. Optimization of Fermentation Processes
3.2.1. RSM Optimization of Dilute Acid Pretreatment
3.2.2. RSM Optimization of Enzymatic Hydrolysis
3.2.3. RSM Optimization of Fermentation
3.3. Monitoring Copper within Hydrolysates
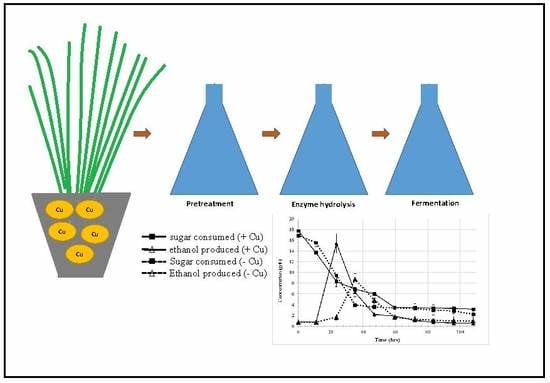
3.4. Effects of Copper Exposure on Ethanol Yield
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
References
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Benedict, C.H. Lake Superior Milling Practice; Michigan College of Mining and Technology Press: Houghton, MI, USA, 1955. [Google Scholar]
- Li, B.; Hwant, J.Y.; Drelich, J.; Popko, D.; Bagley, S. Physical, Chemical and Antimicrobial Characterization of Copper-bearing Material. J. Min. Met. Mat. Soc. 2010, 62, 80–85. [Google Scholar] [CrossRef]
- Rasmussen, T.; Fraser, R.; Lemberg, D.S.; Regis, R. Map** Stamp Sand Dynamics: Gay, Michigan. J. Great Lakes Res. 2002, 28, 276–284. [Google Scholar] [CrossRef]
- Richards, B.K.; Stoof, C.R.; Cary, I.J. Reporting on marginal lands for bioenergy feedstock production: A Modest Proposal. Bioenerg. Res. 2014, 7, 1060–1062. [Google Scholar] [CrossRef]
- Angle, J.S.; Linacre, N.A. Ecological Risks of Novel Environmental Crop Technologies Using Phytoremediation as an Example; Institute, I.F.P.R.: Washington, DC, USA, 2005. [Google Scholar]
- Antiochia, R.; Campanella, L.; Ghezzi, P.; Movassaghi, K. The use of vetiver for remediation of heavy metal soil contamination. Anal. Bioanal. Chem. 2007, 388, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Andra, S.; Datta, R.; Sarkar, D.; Makris, K.C.; Mullens, C.P.; Sahi, S.; Bach, S.B.H. Phytochelatins in Vetiver Grass [Vetiveria zizanioides (L.)]. J. Environ. Qual. 2009, 38, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, Z.; Li, X. The use of vetiver grass (Vetiveria zizanoides) in the phytoremediation of soils contaminated with heavy metals. Appl. Geochem. 2004, 19, 1553–1565. [Google Scholar] [CrossRef]
- Danh, L.T.; Truong, P.; Mammucari, R.; Pu, Y.; Foster, N.R. Phytoremediation of soils contaminated by heavy metals, metalloids, and radioactive materials using vetiver grass, Chrysopogon zizanioides. In Phytotechnologies: Remediation of Environmental Contaminants; CRC Press: Boca Raton, FL, USA, 1996; pp. 255–275. [Google Scholar]
- Kumar, A.; Prasad, R. Production of renewable energy and waste water management from vetiver grass. In Management of Water, Energy, and Bio-Resources in the Era of Climate Change: Emerging Issues and Challenges; Springer International Publishing: Cham, Switzerland, 2015; pp. 169–181. [Google Scholar]
- Dee**g, S.; Ketkorn, W. Comparison of hydrolysis conditions to recover reducing sugar from various lignocellulosic materials. Chain. Mai. J. Sci. 2009, 36, 384–394. [Google Scholar]
- Wongwatanapaiboon, J.; Kangvansaichol, K.; Burapatana, V.; Inochanon, R.; Winayanuwattikun, P.; Yongvanich, T.; Chulalaksananukul, W. The potential of cellulosic ethanol production from grasses in Thailand. J. Biomed. Biotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Chaikumpollert, O.; Methacanon, P.; Suchiva, K. Structural elucidation of hemicelluloses from vetiver grass. Carbohydr. Polym. 2004, 57, 191–196. [Google Scholar] [CrossRef]
- Methacanon, P.; Chaikumpollert, O.; Thavorniti, P.; Suchiva, K. Hemicellulose polymer from vetiver grass and its physiochemical properties. Carbohydr. Polym. 2003, 54, 335–342. [Google Scholar] [CrossRef]
- Arundale, R.A.; Baur, S.; Haffner, F.B.; Mitchell, V.D.; Voigt, T.B.; Long, S.P. Environment has little effect on biomass and biochemical composition of Miscanthus x giganteus across soil types, nitrogen fertilization, and times of harvest. Bioenergy Res. 2015, 8, 1636–1646. [Google Scholar] [CrossRef]
- Ioelovich, M.; Morag, E. Study of enzymatic hydrolysis of mild pretreated lignocellulosic biomasses. Bioresources 2012, 7, 1040–1052. [Google Scholar] [CrossRef]
- Topp, G.C. Soil Sampling and Methods of Analysis; Lewis Publishers: Boca Raton, FL, USA, 1997; pp. 541–543. [Google Scholar]
- Ball, D.F. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Harding, D.E.; Ross, D.J. Some factors in low-temperature storage influencing the mineralisable nitrogen of soils. J. Sci. Food Agric. 1964, 15, 829–834. [Google Scholar] [CrossRef]
- USEPA. Method 1311, Toxicity Characteristic Leaching Procedure (TCLP). Publication SW−846: Test Methods for Evaluating Solid Waste, Physical/Chemical Methods. 1992. Available online: https://www.epa.gov/hw-sw846/sw-846-test-method-1311-toxicity-characteristic-leaching-procedure (accessed on 12 April 2019).
- Sparks, D.L. Selenium and Arsenic. In Methods of Soil Analysis; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1996; pp. 793–832. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Vila, M.; Mehier, S.; Lorber-Pascal, S.; Laurent, F. Phytotoxicity to and uptake of RDX by rice. Environ. Pollut. 2007, 145, 813–817. [Google Scholar] [CrossRef]
- USEPA. Test Methods for Evaluating Solid Waste; Government, U.S. Superintendent of Documents: Washington, DC, USA, 1986.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Foster, C.E.; Martin, T.M.; Pauly, M. Comprehensive compositional analysis of plant cell walls (lignocellulosic biomass) Part II: Carbohydrate analysis. J. Vis. Exper. 2010, 37, e1837. [Google Scholar] [CrossRef]
- O’Connor, G.A.; Sarkar, D.; Brinton, S.R.; Elliot, H.A.; Martin, F.G. Phytoavailability of Biosolids Phosphorus. J. Environ. Qual. 2004, 33, 703–712. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Baltimore, MD, USA, 1992. [Google Scholar]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. Monitoring of contaminated toxic and heavy metals, from mine tailings through age accumulation, in soil and some wild plants at Southeast Egypt. J. Hazar. Mat. 2010, 178, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Minitab, I. Designing an experiment. Get. Started Minitab 2010, 17, 41–52. [Google Scholar]
- Groves, S.L. Optimization of Ethanol Production by Yeasts from Lignocellulosic Feedstocks. Master’s Thesis, Biological Sciences, Michigan Technological University, Houghton, MI, USA, 2010. [Google Scholar]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; National Renewable Energy Laboratory-US Department of Energy: Golden, CO, USA, 2008; NREL/TP-510-42629.
- Sigechi, H.; Koh, J.; Fujita, Y.; Matsumoto, T.; Bito, Y.; Uea, M.; Satoh, E.; Fukuda, H.; Kondo, A. Direct production of ethanol from raw corn starch via fermentation by use of a novel surface-engineered yeast strain codisplaying glucoamylase and alpha-amylase. Appl. Environ. Microbiol. 2004, 70, 5037–5040. [Google Scholar] [CrossRef] [PubMed]
- Bird, M.; Kracht, O.; Derrien, D.; Zhou, Y. The effect of soil texture and roots on the stable carbon isotope composition of soil organic carbon. Soil Res. 2003, 41, 77–94. [Google Scholar] [CrossRef]
- Carter, M.R. Soil quality for sustainable land management. Agron. J. 2002, 94, 38–47. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Vogel, H.; Kasper, B. Mine soils on abandoned gold mine tailing in Francistown North-East District, Republic of Botswana. Depart. Geological Survey, Botswana. 2002. Available online: http://www.limpopo.riverawarenesskit.org/limpoporak_com/_system/dmsstorage/3471en/mine_soils_ftown_sec.pdf (accessed on 12 April 2019).
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant-soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- Llorens, N.; Arola, L.; Blade, C.; Mas, A. Effects of copper exposure upon nitrogen metabolism in tissue cultured Vitis vinifera. Plant Sci. 2000, 160, 159–163. [Google Scholar] [CrossRef]
- Batool, R.; Hameed, M.; Ashraf, M.; Sajid, M.; Ahmad, A.; Fatima, S. Physio-anatomical responses of plants to heavy metals. In Phytoremediation for Green Energy; Springer: Dordrecht, The Netherlands, 2015; pp. 79–96. [Google Scholar]
- Maksymiec, W. Effect of copper on cellular processes in higher plants. Photosynthetica 1997, 34, 321–342. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants; Gulf Professional Publishing: Houston, TX, USA, 1995. [Google Scholar]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal induced reactive oxygen species: Phytotoxicity and physiochemical changes in plants. Rev. Env. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Jiang, L.; Shi, G.; Ding, Y.; Lou, L.; Cai, Q. Differential responses of two bamboo species (Phyllostachys auresulcata ‘Spectabilis’ and Pleioblastus chino ‘Hisauchii’) to excess copper. BioEnergy Res. 2013, 6, 1223–1229. [Google Scholar] [CrossRef]
- Von Wettstein, D.; Gough, S.; Kannangara, G. Chlorophyll biosynthesis. Plant Cell 1995, 7, 1039–1057. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.E.; Ortega-Villasante, C.; Montero-Palmero, M.B.; Escobar, C.; Carpena, R.O. Heavy metal perception in a microscale environment: A model using high doses of pollutants. In Metal Toxicity in Plants: Perception, Signaling, and Remediation; Springer: Heidelberg, Germany, 2012; pp. 23–39. [Google Scholar]
- McIntyre, T. Plant adaptations to soils contaminated with metal. In Phytoremediation; Springer: New York, NY, USA, 2003; pp. 103–108. [Google Scholar]
- Bhardwaj, B. Physiological Mechanisms and Adaptation Strategies in Plants under Changing Environment; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Lorenzo, M.; Moldes, D.; Sanroman, M.A. Effect of heavy metals on the production of several laccase isoenzymes by Trametes versicolor and on their ability to decolourise dyes. Chemosphere 2006, 63, 912–917. [Google Scholar] [CrossRef] [PubMed]
- Kannaiyan, R.; Mahinpey, N.; Mani, T.; Martinuzzi, R.J.; Kostenko, V. Enhancement of Dichomitus squalens tolerance to copper and copper-associated laccase activity by carbon and nitrogen sources. Biochem. Eng. J. 2012, 67, 140–147. [Google Scholar] [CrossRef]
- Hilden, K.; Makela, M.R.; Lankinen, P.; Lundell, T. Agaricus bisporus and related Agaricus species on lignocellulose: Production of manganese peroxidase and multicopper oxidases. Fungal Genet. Biol. 2013, 55, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Cara, C.; Ruiz, E.; Oliva, J.M.; Saez, F.; Castro, E. Conversion of olive tree biomass into fermentable sugars by dilute acid pretreatment and enzymatic saccharfication. Bioresour. Technol. 2008, 99, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.; Palmqvist, E.; Hahn-Hagerdal, B.; Tengborg, C.; Galbe, M.; Zacchi, G.; Stenberg, K.; Zacchi, G.; Nilvenbrant, N.O. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym. Microb. Technol. 1999, 23, 151–159. [Google Scholar] [CrossRef]
- Banerjee, N.; Bhatnagar, R.; Viswanathan, L. Inhibition of glycolysis by furfural in Saccharomyces cerevisiae. Eur. J. Appl. Microbiol. Biotechnol. 1981, 11, 226–228. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Hendrickson, R.; Nathan, S.M.; Michael, R.L.; Balan-Venkatesh, B.; Bruce, E.D. Enzyme hydrolysis and ethanol fermentation of liquid hot water and AFEX pretreated distiller’s grains at high solid loadings. Bioresour. Technol. 2008, 99, 5206–5215. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.L.; Chen, W.H.; Chen, W.H.; Men, L.C.; Hwang, W.S. Characterization of dilute acid pretreatment of silvergrass for ethanol production. Bioresour. Technol. 2008, 99, 6046–6053. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Kuttiraja, M.; Binod, P.; Janu, K.U.; Sukumaran, R.K.; Pandey, A. Dilute acid pretreatment and enzymatic saccharification of sugarcane tops for bioethanol production. Bioresour. Technol. 2011, 102, 10915–10921. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.T.; Siu, R.G.H.; Levinson, H.S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J. Bacteriol. 1950, 59, 485–497. [Google Scholar] [PubMed]
- Jeya, M.; Zhang, Y.W.; Kim, I.W.; Lee, J.K. Enhanced saccharification of alkali-treated rice straw by cellulase from Trametes hirsuta and statistical optimization of hydrolysis conditions by RSM. Bioresour. Technol. 2009, 100, 5155–5161. [Google Scholar] [CrossRef]
- Kunamneni, A.; Singh, S. Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem. Eng. J. 2005, 27, 179–190. [Google Scholar] [CrossRef]
- Tengborg, C.; Galbe, M.; Zacchi, G. Influence of enzyme loading and physical parameteres on the enzymatic hydrolysis of steam-pretreated softwood. Biotechnol. Prog. 2001, 17, 110–117. [Google Scholar] [CrossRef]
- Santos, S.C.; Dionisio, S.R.; DeAndrade, A.L.D.; Roque, L.R.; DaCosta, A.C.; Ienczak, J.L. Fermentation of xylose and glucose mixture in intensified reactors by Scheffersomyces stipitis to produce ethanol. Int. J. Biol. Biomol. Agri. Food Biotechnol. Eng. 2015, 9, 503–508. [Google Scholar]
- Scordia, D.; Cosentino, S.L.; Lee, J.W.; Jeffries, T.W. Bioconversion of giant reed (Arundo donax L.) hemicellulose hydrolysate to ethanol by Scheffersomyces stipitis CBS6054. Biomass Bioenergy 2012, 39, 296–305. [Google Scholar] [CrossRef]
- Sharma, S.K.; Kalra, K.L.; Kocher, G.S. Fermentation of enzymatic hydrolysate of sunflower hulls for ethanol production and its scale up. Biomass Bioenergy 2004, 27, 399–402. [Google Scholar] [CrossRef]
- Unrean, P.; Nguyen, N.H.A. Rational optimization of culture conditions for the most effiecient ethanol production in Scheffersomyces stipits using design of experiments. Biotechnol. Prog. 2012, 28, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.S.; Lee, Y.Y. Ethanol fermentation of crude acid hydrolyzate of cellulose using high-level yeast inocula. Biotechnol. Bioeneg. 2004, 27, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Micobiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef]
- De Freitas, J.; Wintz, H.; Kim, J.H.; Poynton, H.; Fox, T.; Vulpe, C. Yeast, a model organism for iron and copper metabolism studies. Biometals 2003, 16, 185–197. [Google Scholar] [CrossRef]
- Stehlik-Thomas, V.; Grba, S.; Stanzer, D.; Vahcic, N.; Zetic, V.G. Uptake of iron by yeast cells and its impact on biomass production. Acta Aliment. 2003, 32, 279–287. [Google Scholar] [CrossRef]
- Soderstrom, J.; Pilcher, L.; Galbe, M.; Zacchi, G. Two-step pretreatment of softwood by dilute H2SO4 impregnation for ethanol production. Biomass Bioenerg. 2003, 24, 475–486. [Google Scholar] [CrossRef]
- CARD Research Biorenewables Policy-Historical Ethanol Operating Margins. Available online: https://www.card.iastate.edu/research/biorenewables/tools/hist_eth_gm.aspx (accessed on 24 May 2019).
- Dhiman, S.; Selvaraj, C.; Li, J.; Singh, R.; Zhao, X.; Kim, D.; Kim, R.; Kang, Y.; Lee, J. Phytoremediation of metal-contaminated soils by the hyperaccumulator canola (Brassica napus L.) and the use of its biomass for ethanol production. Fuel 2016, 183, 107–114. [Google Scholar] [CrossRef]
- Cheng, S.-F.; Huang, C.-Y.; Chen, K.-L.; Lin, S.-C.; Lin, Y.-C. Exploring the benefits of growing bioenergy crops to activate lead-contaminated agricultural land: A case study on sweet potatoes. Environ. Monit. Assess. 2015, 187, 144. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-H.; Yu, F.C.; Chang, F.-C.; Yang, B.-Y.; Chen, W.-H.; Hwang, W.-S.; Tu, T.-C. Bioethanol production from recovered napier grass with heavy metals. J. Environ. Manag. 2017, 203, 1005–1110. [Google Scholar] [CrossRef] [PubMed]




| Stamp Sand | Control Soil | |||
| Particle Size Distribution | % Clay | 6.23 ± 2.36 | 15.20 ± 0.06 | |
| % Silt | 3.77 ± 0.80 | 10.20 ± 0.08 | ||
| % Sand Total | 69.74 ± 1.28 | 70.81 ± 0.09 | ||
| % Sand 1000 μm | 4.48 ± 1.15 | 0 ± 0 | ||
| % Sand 500 μm | 14.45 ± 0.95 | 1.29 ± 0.05 | ||
| % Sand 400 μm | 12.06 ± 0.16 | 1.70 ± 0.02 | ||
| % Sand 250 μm | 38.76 ± 1.45 | 85.75 ± 0.03 | ||
| % Course silt | 20.25 ± 1.88 | 5.01 ± 0.02 | ||
| pH | 6.82 ± 0.02 | 5.44 ± 0.02 | ||
| Electrical conductivity (μS) | 12.7 ± 0.21 | 20.9 ± 1.23 | ||
| Water holding capacity (% WHC) | 32.12 ± 1.44 | 29.7 ± 0.01 | ||
| Water content (%) | 22.96 ± 2.63 | 46.01 ± 0.01 | ||
| Organic matter content (% LOI) | 0.92 ± 0.20 | 3.45 ± 0.12 | ||
| Tamm’s Reagent (mg/kg) | Al | 22.45 ± 0.04 | 11.65 ± 0.01 | |
| Fe | 89.00 ± 0.02 | 93.97 ± 0.94 | ||
| Toxicity Characteristic Leaching Procedure (mg/kg) | Cu | 394.32 ± 118.18 | 96.55 ± 73.43 | |
| Fe | 47.20 ± 13.94 | 64.20 ± 12.69 | ||
| Al | 252.7 ± 28.22 | 39.27 ± 13.65 | ||
| Total Metal Analysis (mg/kg) | Cu | 1551 ± 28.28 | 27.05 ± 9.68 | |
| Fe | 44830 ± 806.00 | 12212.5 ± 333.08 | ||
| Al | 7586.62 ± 761.88 | 3777.52 ± 558.34 | ||
| Sequential Extraction (mg/kg) | Acid-soluble | Cu | 397.37 ± 3.18 | BDL |
| Reducible | Cu | 367.00 ± 1.18 | BDL | |
| Oxidizable | Cu | 126.60 ± 2.94 | BDL | |
| Residual | Cu | 1150.67 ± 10.26 | 0.65 ± 0.09 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiger, E.M.; Sarkar, D.; Datta, R. Evaluation of Copper-Contaminated Marginal Land for the Cultivation of Vetiver Grass (Chrysopogon zizanioides) as a Lignocellulosic Feedstock and its Impact on Downstream Bioethanol Production. Appl. Sci. 2019, 9, 2685. https://doi.org/10.3390/app9132685
Geiger EM, Sarkar D, Datta R. Evaluation of Copper-Contaminated Marginal Land for the Cultivation of Vetiver Grass (Chrysopogon zizanioides) as a Lignocellulosic Feedstock and its Impact on Downstream Bioethanol Production. Applied Sciences. 2019; 9(13):2685. https://doi.org/10.3390/app9132685
Chicago/Turabian StyleGeiger, Emily M., Dibyendu Sarkar, and Rupali Datta. 2019. "Evaluation of Copper-Contaminated Marginal Land for the Cultivation of Vetiver Grass (Chrysopogon zizanioides) as a Lignocellulosic Feedstock and its Impact on Downstream Bioethanol Production" Applied Sciences 9, no. 13: 2685. https://doi.org/10.3390/app9132685