Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
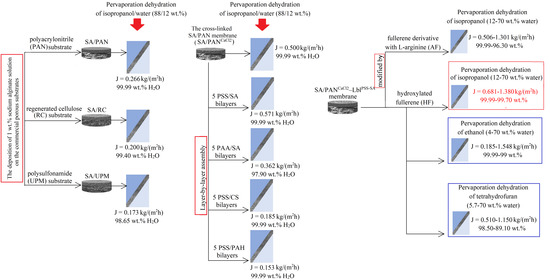
2.2.1. Supported Membranes
2.2.2. Surface Modification with Layer-by-Layer (Lbl) Assembly
2.3. Pervaporation
2.4. Scanning Electron Microscopy (SEM)
2.5. Atomic Force Microscopy (AFM)
2.6. The Standard Porosimetry Method
2.7. Filtration Performance of Substrates
2.8. Contact Angle Measurement
3. Results
3.1. The Development of the Supported SA Membranes
3.1.1. Transport Properties of the Supported SA Membranes
3.1.2. The Investigation of the Substrates
3.2. Surface Modification of the Supported SA and SA/Fullerene Derivative Membranes by Lbl Deposition of PEL
3.2.1. The Investigation of Membranes Based on Parent SA
3.2.2. The Investigation of Membranes Based on SA/Fullerene Derivative Composites
3.3. Comparison of the Performance of the Membranes with PEL Layers with Membranes Described in the Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2020, 11, 4. [Google Scholar] [CrossRef]
- Lei, B.; Peng, M.; Liu, L.; Hu, S.; Zhang, W.; Meng, G. Galvanic Corrosion Performance of an Al–BN Abradable Seal Coating System in Chloride Solution. Coatings 2020, 11, 9. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Q.; Li, Y.; Yang, Y.; Zhang, T.; Wang, Y.; Shao, Y.; Sun, H.; Wang, Z.; Wang, F. Optimization of the Corrosion Resistance of Electroless Ni–W–P Coatings on Magnesium Alloys by the Response Surface Methodology. Coatings 2020, 11, 18. [Google Scholar] [CrossRef]
- Daroonparvar, M.; Kasar, A.K.; Farooq Khan, M.U.; Menezes, P.L.; Kay, C.M.; Misra, M.; Gupta, R.K. Improvement of Wear, Pitting Corrosion Resistance and Repassivation Ability of Mg-Based Alloys Using High Pressure Cold Sprayed (HPCS) Commercially Pure-Titanium Coatings. Coatings 2021, 11, 57. [Google Scholar] [CrossRef]
- Aly, K.I.; Mahdy, A.; Hegazy, M.A.; Al-Muaikel, N.S.; Kuo, S.-W.; Gamal Mohamed, M. Corrosion Resistance of Mild Steel Coated with Phthalimide-Functionalized Polybenzoxazines. Coatings 2020, 10, 1114. [Google Scholar] [CrossRef]
- Jmiai, A.; El Ibrahimi, B.; Tara, A.; El Issami, S.; Jbara, O.; Bazzi, L. Alginate biopolymer as green corrosion inhibitor for copper in 1 M hydrochloric acid: Experimental and theoretical approaches. J. Mol. Struct. 2018, 1157, 408–417. [Google Scholar] [CrossRef]
- Cirule, D.; Sansonetti, E.; Andersone, I.; Kuka, E.; Andersons, B. Enhancing Thermally Modified Wood Stability against Discoloration. Coatings 2021, 11, 81. [Google Scholar] [CrossRef]
- Claver, A.; Jiménez-Piqué, E.; Palacio, J.F.; Almandoz, E.; Fernández de Ara, J.; Fernández, I.; Santiago, J.A.; Barba, E.; García, J.A. Comparative Study of Tribomechanical Properties of HiPIMS with Positive Pulses DLC Coatings on Different Tools Steels. Coatings 2020, 11, 28. [Google Scholar] [CrossRef]
- Qiang, X.; Guo, X.; Quan, Q.; Su, H.; Huang, D. Improving the Adsorption Performance of Loofah Sponge towards Methylene Blue by Coating Ca2+ Crosslinked Sodium Alginate Layers on Its Fiber Surface. Coatings 2020, 10, 814. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Z.-C.; Lin, C.-J. Preparation and characterization of nano-sized hydroxyapatite particles and hydroxyapatite/chitosan nano-composite for use in biomedical materials. Mater. Lett. 2002, 57, 858–861. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrophoretic deposition of composite hydroxyapatite-chitosan coatings. Mater. Charact. 2007, 58, 339–348. [Google Scholar] [CrossRef]
- Bumgardner, J.D.; Wiser, R.; Gerard, P.D.; Bergin, P.; Chestnutt, B.; Marini, M.; Ramsey, V.; Elder, S.H.; Gilbert, J.A. Chitosan: Potential use as a bioactive coating for orthopaedic and craniofacial/dental implants. J. Biomater. Sci. Polym. Ed. 2003, 14, 423–438. [Google Scholar] [CrossRef]
- Santoro, S.; Drioli, E.; Figoli, A. Development of Novel ECTFE Coated PP Composite Hollow-Fiber Membranes. Coatings 2016, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, X.; Qi, M.; Bai, T.; Zhao, K.; Zhang, X. Removal of Dyes and Cd2+ in Water by Kaolin/Calcium Alginate Filtration Membrane. Coatings 2019, 9, 218. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Tao, Z.; Chen, L.; Han, M.; Zhao, B.; Tian, X.; Wang, L.; Meng, F. An antifouling catechol/chitosan-modified polyvinylidene fluoride membrane for sustainable oil-in-water emulsions separation. Front. Environ. Sci. Eng. 2021, 15, 63. [Google Scholar] [CrossRef]
- Wai, K.P.; Koo, C.H.; Pang, Y.L.; Chong, W.C.; Lau, W.J. In situ immobilization of silver on polydopamine-coated composite membrane for enhanced antibacterial properties. J. Water Process Eng. 2020, 33, 100989. [Google Scholar] [CrossRef]
- Ursino, C.; Ounifi, I.; Di Nicolò, E.; Cheng, X.Q.; Shao, L.; Zhang, Y.; Drioli, E.; Criscuoli, A.; Figoli, A. Development of non-woven fabric-based ECTFE membranes for direct contact membrane distillation application. Desalination 2021, 500, 114879. [Google Scholar] [CrossRef]
- Bassil, J.; Alem, H.; Henrion, G.; Roizard, D. Tailored adhesion behavior of polyelectrolyte thin films deposited on plasma-treated poly(dimethylsiloxane) for functionalized membranes. Appl. Surf. Sci. 2016, 369, 482–491. [Google Scholar] [CrossRef]
- Wu, J.; Hou, Z.; Yu, Z.; Lang, J.; Cui, J.; Yang, J.; Dai, J.; Li, C.; Yan, Y.; **e, A. Facile preparation of metal-polyphenol coordination complex coated PVDF membrane for oil/water emulsion separation. Sep. Purif. Technol. 2021, 258, 118022. [Google Scholar] [CrossRef]
- Salehian, P.; Chung, T.-S. Two-dimensional (2D) particle coating on membranes for pervaporation dehydration of isopropanol: A new approach to seal defects and enhance separation performance. J. Membr. Sci. 2017, 544, 378–387. [Google Scholar] [CrossRef]
- Wu, H.; Lu, X.; Li, X.; Li, Y.; Zhao, C.; Jiang, Z. Enhancing structural stability and pervaporation performance of composite membranes by coating gelatin onto hydrophilically modified support layer. Chin. J. Chem. Eng. 2014, 22, 19–27. [Google Scholar] [CrossRef]
- Page, C.A.; Fouda, A.E.; Matsuura, T. Pervaporation performance of polyetherimide membranes spin- and dip-coated with polydimethylsiloxane. J. Appl. Polym. Sci. 1994, 54, 975–989. [Google Scholar] [CrossRef]
- Peters, T.A.; Benes, N.E.; Keurentjes, J.T.F. Zeolite-Coated Ceramic Pervaporation Membranes; Pervaporation−Esterification Coupling and Reactor Evaluation. Ind. Eng. Chem. Res. 2005, 44, 9490–9496. [Google Scholar] [CrossRef]
- Ong, Y.K.; Wang, H.; Chung, T. A prospective study on the application of thermally rearranged acetate-containing polyimide membranes in dehydration of biofuels via pervaporation. Chem. Eng. Sci. 2012, 79, 41–53. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Davey, C.J.; Leak, D.; Patterson, D.A. Hybrid and Mixed Matrix Membranes for Separations from Fermentations. Membranes 2016, 6, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced Pervaporation Properties of PVA-Based Membranes Modified with Polyelectrolytes. Application to IPA Dehydration. Polymers 2019, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Yushkin, A.A.; Efimov, M.N.; Malakhov, A.O.; Karpacheva, G.P.; Bondarenko, G.; Marbelia, L.; Vankelecom, I.F.J.; Volkov, A.V. Creation of highly stable porous polyacrylonitrile membranes using infrared heating. React. Funct. Polym. 2021, 158, 104793. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Hsu, C.-Y.; Huang, S.-H.; Lee, K.-R.; Hung, W.-S.; Hu, C.-C.; Lai, J.-Y. The preparation of polyelectrolyte/hydrolyzed polyacrylonitrile composite hollow fiber membrane for pervaporation. J. Taiwan Inst. Chem. Eng. 2018, 91, 623–633. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhim, J.W.; Feng, X. Improving the stability of layer-by-layer self-assembled membranes for dehydration of alcohol and diol. J. Membr. Sci. 2013, 444, 22–31. [Google Scholar] [CrossRef]
- Ge, L.; Wu, L.; Wu, B.; Wang, G.; Xu, T. Preparation of monovalent cation selective membranes through annealing treatment. J. Membr. Sci. 2014, 459, 217–222. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kwon, Y.; Shon, M.; Nam, S. Journal of Industrial and Engineering Chemistry Surface-modi fi ed polyvinyl alcohol ( PVA ) membranes for pervaporation dehydration of epichlorohydrin ( ECH ), isopropanol ( IPA ), and water ternary feed mixtures. J. Ind. Eng. Chem. 2020, 81, 185–195. [Google Scholar] [CrossRef]
- Lee, J.-Y.; She, Q.; Huo, F.; Tang, C.Y. Metal–organic framework-based porous matrix membranes for improving mass transfer in forward osmosis membranes. J. Membr. Sci. 2015, 492, 392–399. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Pan, F.; He, G.; Fang, C.; Cao, K.; **ng, R.; Jiang, Z. Fabricating graphene oxide-based ultrathin hybrid membrane for pervaporation dehydration via layer-by-layer self-assembly driven by multiple interactions. J. Membr. Sci. 2015, 487, 162–172. [Google Scholar] [CrossRef]
- Yu, J.; Cheng, S.; Che, Q. Preparation and characterization of layer-by-layer self-assembly membrane based on sulfonated polyetheretherketone and polyurethane for high-temperature proton exchange membrane. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3446–3454. [Google Scholar] [CrossRef]
- Lefaux, C.J.; Kim, B.-S.; Venkat, N.; Mather, P.T. Molecular Composite Coatings on Nafion Using Layer-by-Layer Self-Assembly. ACS Appl. Mater. Interfaces 2015, 7, 10365–10373. [Google Scholar] [CrossRef]
- Zuin, S.; Scanferla, P.; Brunelli, A.; Marcomini, A.; Wong, J.E.; Wennekes, W.; Genné, I. Layer-by-Layer Deposition of Titanium Dioxide Nanoparticles on Polymeric Membranes: A Life Cycle Assessment Study. Ind. Eng. Chem. Res. 2013, 52, 13979–13990. [Google Scholar] [CrossRef]
- Dragan, E.S.; Mihai, M.; Schauer, J.; Ghimici, L. PAN composite membrane with different solvent affinities controlled by surface modification methods. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4161–4171. [Google Scholar] [CrossRef]
- Joseph, N.; Ahmadiannamini, P.; Hoogenboom, R.; Vankelecom, I.F.J. Layer-by-layer preparation of polyelectrolyte multilayer membranes for separation. Polym. Chem. 2014, 5, 1817–1831. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Penkova, A.; Kuzminova, A.; Missyul, A.; Ermakov, S.; Roizard, D. Development and characterization of new pervaporation PVA membranes for the dehydration using bulk and surface modifications. Polymers 2018, 10, 571. [Google Scholar] [CrossRef] [Green Version]
- Dmitrenko, M.E.; Penkova, A.V.; Kuzminova, A.I.; Morshed, M.; Larionov, M.I.; Alem, H.; Zolotarev, A.A.; Ermakov, S.S.; Roizard, D. Investigation of new modification strategies for PVA membranes to improve their dehydration properties by pervaporation. Appl. Surf. Sci. 2018, 450, 527–537. [Google Scholar] [CrossRef]
- Tieke, B.; van Ackern, F.; Krasemann, L.; Toutianoush, A. Ultrathin self-assembled polyelectrolyte multilayer membranes. Eur. Phys. J. E 2001, 5, 29–39. [Google Scholar] [CrossRef]
- Klitzing, R.; Tieke, B. Polyelectrolyte Membranes; Springer: Berlin/Heidelberg, Germany, 2004; pp. 177–210. [Google Scholar]
- Krasemann, L.; Tieke, B. Ultrathin self-assembled polyelectrolyte membranes for pervaporation. J. Membr. Sci. 1998, 150, 23–30. [Google Scholar] [CrossRef]
- De Guzman, M.R.; Belle, M.; Yap, M.; Huang, S.; Jhuang, W.; Tsai, H.; Hu, C.; Lee, K.; Lai, J. Layer-by-layer self-assembly of polyethyleneimine and poly (4-styrene sulfonic acid-co-maleic acid ) forming composite polyelectrolyte membranes for pervaporation of aqueous alcohol solutions. J. Polym. Res. 2019, 26, 286. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Lahderanta, E.; Ermakov, S.; Penkova, A. Mixed matrix membranes based on sodium alginate modified by fullerene derivatives with L-amino acids for pervaporation isopropanol dehydration. J. Mater. Sci. 2021, 56, 7765–7787. [Google Scholar] [CrossRef]
- Dudek, G.; Krasowska, M.; Turczyn, R.; Gnus, M.; Strzelewicz, A. Structure, morphology and separation efficiency of hybrid Alg/Fe3O4 membranes in pervaporative dehydration of ethanol. Sep. Purif. Technol. 2017, 182, 101–109. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Ghaffar, A.; Huang, Q. Development and characterization of sodium alginate/poly(sodium 4-styrenesulfonate) composite films for release behavior of ciprofloxacin hydrogen chloride monohydrate. Polym. Polym. Compos. 2021, 96739112199027. [Google Scholar] [CrossRef]
- Krasemann, L.; Toutianoush, A.; Tieke, B. Self-assembled polyelectrolyte multilayer membranes with highly improved pervaporation separation of ethanol/water mixtures. J. Membr. Sci. 2001, 181, 221–228. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, permeance and selectivity: A preferred way of reporting pervaporation performance data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Sampranpiboon, P.; Jiraratananon, R.; Uttapap, D.; Feng, X.; Huang, R.Y. Pervaporation separation of ethyl butyrate and isopropanol with polyether block amide (PEBA) membranes. J. Membr. Sci. 2000, 173, 53–59. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Rajabi, L.; Derakhshan, A.A.; Seyedpour, F. PAA grafting onto new acrylate-alumoxane/PES mixed matrix nano-enhanced membrane: Preparation, characterization and performance in dye removal. Chem. Eng. J. 2013, 221, 111–123. [Google Scholar] [CrossRef]
- Naik, P.V.; Bernstein, R.; Vankelecom, I.F.J. Influence of support layer and PDMS coating conditions on composite membrane performance for ethanol/water separation by pervaporation. J. Appl. Polym. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Hao, P. Influence of the porous support on diffusion in composite membranes. J. Membr. Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Sazanova, T.S.; Otvagina, K.V.; Kryuchkov, S.S.; Zarubin, D.M.; Fukina, D.G.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Revealing the Surface Effect on Gas Transport and Mechanical Properties in Nonporous Polymeric Membranes in Terms of Surface Free Energy. Langmuir 2020, 36, 12911–12921. [Google Scholar] [CrossRef] [PubMed]
- Kosvintsev, S.; Cumming, I.; Holdich, R.; Lloyd, D.; Starov, V. Sieve mechanism of microfiltration separation. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 230, 167–182. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology, 2nd ed.; Springer Netherlands: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Plisko, T.V.; Penkova, A.V.; Burts, K.S.; Bildyukevich, A.V.; Dmitrenko, M.E.; Melnikova, G.B.; Atta, R.R.; Mazur, A.S.; Zolotarev, A.A.; Missyul, A.B. Effect of Pluronic F127 on porous and dense membrane structure formation via non-solvent induced and evaporation induced phase separation. J. Membr. Sci. 2019, 580, 336–349. [Google Scholar] [CrossRef]
- Anokhina, T.; Borisov, I.; Yushkin, A.; Vaganov, G.; Didenko, A.; Volkov, A. Phase Separation within a Thin Layer of Polymer Solution as Prompt Technique to Predict Membrane Morphology and Transport Properties. Polymers 2020, 12, 2785. [Google Scholar] [CrossRef]
- Kamal, N.; Kochkodan, V.; Zekri, A.; Ahzi, S. Polysulfone Membranes Embedded with Halloysites Nanotubes: Preparation and Properties. Membranes 2019, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Rubner, M.F. Influence of the Degree of Ionization on Weak Polyelectrolyte Multilayer Assembly. Macromolecules 2005, 38, 116–124. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on Pervaporation: Theory, Membrane Performance, and Application to Intensification of Esterification Reaction. J. Eng. 2015, 2015, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-J.; Ramaswamy, S.; Liu, Y. Separation and purification of biobutanol during bioconversion of biomass. Sep. Purif. Technol. 2014, 132, 513–540. [Google Scholar] [CrossRef]
- Huang, Y.; Baker, R.W.; Vane, L.M. Low-Energy Distillation-Membrane Separation Process. Ind. Eng. Chem. Res. 2010, 49, 3760–3768. [Google Scholar] [CrossRef]
- Horsley, L.H. Azeotropic Data—III. In Advances in Chemistry Series 116; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1973; pp. 15; 18. [Google Scholar]
- Koczka, K.; Manczinger, J.; Mizsey, P.; Fonyo, Z. Novel hybrid separation processes based on pervaporation for THF recovery. Chem. Eng. Process. Process Intensif. 2007, 46, 239–246. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Zolotarev, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Ermakov, S.; Penkova, A. Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol. Polymers 2021, 13, 674. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Jeevan Kumar, B.K.; Kittur, A.A.; Kariduraganavar, M.Y. Novel approach for the development of pervaporation membranes using sodium alginate and chitosan-wrapped multiwalled carbon nanotubes for the dehydration of isopropanol. J. Membr. Sci. 2013, 425–426, 77–88. [Google Scholar] [CrossRef]
- Ra**iKanth, V.; Ravindra, S.; Madalageri, P.M.; Kajjari, P.B.; Mulaba-Bafubiandi, A.F. Study of enhanced physical and pervaporation properties in composite membrane. Membr. Water Treat. 2017, 8, 483–498. [Google Scholar]
- Adoor, S.G.; Ra**eekanth, V.; Nadagouda, M.N.; Chowdoji Rao, K.; Dionysiou, D.D.; Aminabhavi, T.M. Exploration of nanocomposite membranes composed of phosphotungstic acid in sodium alginate for separation of aqueous-organic mixtures by pervaporation. Sep. Purif. Technol. 2013, 113, 64–74. [Google Scholar] [CrossRef]
- Sajjan, A.M.; Premakshi, H.G.; Kariduraganavar, M.Y. Synthesis and characterization of polyelectrolyte complex membranes for the pervaporation separation of water–isopropanol mixtures using sodium alginate and gelatin. Polym. Bull. 2018, 75, 851–875. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Pervaporation separation of water-isopropyl alcohol mixtures with blend membranes of sodium alginate and poly(acrylamide)-grafted guar gum. J. Appl. Polym. Sci. 2002, 85, 2014–2024. [Google Scholar] [CrossRef]
- Rachipudi, P.S.; Kittur, A.A.; Sajjan, A.M.; Kamble, R.R.; Kariduraganavar, M.Y. Solving the trade-off phenomenon in separation of water-dioxan mixtures by pervaporation through crosslinked sodium-alginate membranes with polystyrene sulfonic acid-co-maleic acid. Chem. Eng. Sci. 2013, 94, 84–92. [Google Scholar] [CrossRef]
- Patil, M.B.; Veerapur, R.S.; Patil, S.A.; Madhusoodana, C.D.; Aminabhavi, T.M. Preparation and characterization of filled matrix membranes of sodium alginate incorporated with aluminum-containing mesoporous silica for pervaporation dehydration of alcohols. Sep. Purif. Technol. 2007, 54, 34–43. [Google Scholar] [CrossRef]
- Mali, M.G.; Gokavi, G.S. Sorption and permeation studies for isopropanol + water mixtures using alginate based highly water selective nanocomposite membranes. J. Polym. Res. 2012, 19, 9976. [Google Scholar] [CrossRef]
- Maruthi, Y.; Kumara Babu, P.; Subha, M.C.S.; Chowdoji Rao, K. Phosphotungstic acid loaded mixed matrix membranes of sodium alginate karayagum for dehydration of aqueous-organic mixtures. Indian J. Chem. Technol. 2018, 25, 459–467. [Google Scholar]
- Lecaros, R.L.G.; Chua, K.Y.; Tayo, L.L.; Hung, W.-S.; Hu, C.-C.; An, Q.-F.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. The fine-structure characteristics and isopropanol/water dehydration through pervaporation composite membranes improved with graphene quantum dots. Sep. Purif. Technol. 2020, 247, 116956. [Google Scholar] [CrossRef]
- Lecaros, R.L.G.; Bismonte, M.E.; Doma, B.T.; Hung, W.-S.; Hu, C.-C.; Tsai, H.-A.; Huang, S.-H.; Lee, K.-R.; Lai, J.-Y. Alcohol dehydration performance of pervaporation composite membranes with reduced graphene oxide and graphene quantum dots homostructured filler. Carbon N. Y. 2020, 162, 318–327. [Google Scholar] [CrossRef]
- Wei, Y.-M.; Xu, Z.-L.; Qusay, F.A.; Wu, K. Polyvinyl alcohol/polysulfone (PVA/PSF) hollow fiber composite membranes for pervaporation separation of ethanol/water solution. J. Appl. Polym. Sci. 2005, 98, 247–254. [Google Scholar] [CrossRef]
- Wei, P.; Qu, X.; Dong, H.; Zhang, L.; Chen, H.; Gao, C. Silane-modified NaA zeolite/PAAS hybrid pervaporation membranes for the dehydration of ethanol. J. Appl. Polym. Sci. 2013, 128, 3390–3397. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.-H.; Wei, S.-W.; Chiao, Y.-H.; Aquino, R.R.; Hung, W.-S.; Tsai, H.-A.; Lee, K.-R.; Lai, J.-Y. Surface Properties, Free Volume, and Performance for Thin-Film Composite Pervaporation Membranes Fabricated through Interfacial Polymerization Involving Different Organic Solvents. Polymers 2020, 12, 2326. [Google Scholar] [CrossRef]
- An, Q.-F.; Ang, M.B.M.Y.; Huang, Y.-H.; Huang, S.-H.; Chiao, Y.-H.; Lai, C.-L.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Wu, Y.-P.; et al. Microstructural characterization and evaluation of pervaporation performance of thin-film composite membranes fabricated through interfacial polymerization on hydrolyzed polyacrylonitrile substrate. J. Membr. Sci. 2019, 583, 31–39. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.-H.; Chang, M.-W.; Lai, C.-L.; Tsai, H.-A.; Hung, W.-S.; Hu, C.-C.; Lee, K.-R. Ultraviolet-initiated graft polymerization of acrylic acid onto thin-film polyamide surface for improved ethanol dehydration performance of pervaporation membranes. Sep. Purif. Technol. 2020, 235, 116155. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Aroujalian, A.; Raisi, A.; Fotouhi, M. Preparation and characterization of thin film nanocomposite membrane for pervaporative dehydration of aqueous alcohol solutions. Desalination 2013, 314, 20–27. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, G.; Guan, K.; Chen, X.; Chu, Z.; Liu, G.; **, W. Dehydration of C2–C4 alcohol/water mixtures via electrostatically enhanced graphene oxide laminar membranes. AIChE J. 2021. [Google Scholar] [CrossRef]
- **e, H.R.; Ji, C.H.; Xue, S.M.; Xu, Z.L.; Yang, H.; Ma, X.H. Enhanced pervaporation performance of SA-PFSA/ceramic hybrid membranes for ethanol dehydration. Sep. Purif. Technol. 2018, 206, 218–225. [Google Scholar] [CrossRef]
- Villagra Di Carlo, B.; Habert, A.C. Plasma-treated polyethersulfone coated with crosslinked poly(vinyl alcohol): Composite membranes for pervaporation dehydration of ethanol. J. Mater. Sci. 2013, 48, 1457–1464. [Google Scholar] [CrossRef]
- Li, B.-B.; Xu, Z.-L.; Alsalhy Qusay, F.; Li, R. Chitosan-poly (vinyl alcohol)/poly (acrylonitrile) (CS–PVA/PAN) composite pervaporation membranes for the separation of ethanol–water solutions. Desalination 2006, 193, 171–181. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, Y.; Wen, R.; Guo, Y.H.; Su, J.F.; Matsuura, T. Multilayer poly(vinyl alcohol)-zeolite 4A composite membranes for ethanol dehydration by means of pervaporation. Sep. Purif. Technol. 2006, 51, 126–136. [Google Scholar] [CrossRef]
- Das, P.; Ray, S.K. Synthesis and characterization of biopolymer based mixed matrix membranes for pervaporative dehydration. Carbohydr. Polym. 2014, 103, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.G.; Kittur, A.A.; Kariduraganavar, M.Y. Dehydration of THF-water mixtures using zeolite-incorporated polymeric membranes. J. Appl. Polym. Sci. 2008, 111, 2408–2418. [Google Scholar] [CrossRef]
- Chapman, P.; Loh, X.X.; Livingston, A.G.; Li, K.; Oliveira, T.A.C. Polyaniline membranes for the dehydration of tetrahydrofuran by pervaporation. J. Membr. Sci. 2008, 309, 102–111. [Google Scholar] [CrossRef]
- Penkova, A.V.; Dmitrenko, M.E.; Ermakov, S.S.; Toikka, A.M.; Roizard, D. Novel green PVA-fullerenol mixed matrix supported membranes for separating water-THF mixtures by pervaporation. Environ. Sci. Pollut. Res. 2017, 25, 20354–20362. [Google Scholar] [CrossRef] [PubMed]
- Otvagina, K.; Penkova, A.; Dmitrenko, M.; Kuzminova, A.; Sazanova, T.; Vorotyntsev, A.; Vorotyntsev, I. Novel Composite Membranes Based on Chitosan Copolymers with Polyacrylonitrile and Polystyrene: Physicochemical Properties and Application for Pervaporation Dehydration of Tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, P.D.; Tan, X.; Livingston, A.G.; Li, K.; Oliveira, T. Dehydration of tetrahydrofuran by pervaporation using a composite membrane. J. Membr. Sci. 2006, 268, 13–19. [Google Scholar] [CrossRef]
- Ang, M.B.M.Y.; Huang, S.-H.; Li, Y.-C.; Cahatol, A.T.C.; Tayo, L.L.; Hung, W.-S.; Tsai, H.-A.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. High-performance thin-film composite polyetheramide membranes for the dehydration of tetrahydrofuran. J. Membr. Sci. 2020, 611, 118373. [Google Scholar] [CrossRef]
- Huang, S.H.; Liu, Y.Y.; Huang, Y.H.; Liao, K.S.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Study on characterization and pervaporation performance of interfacially polymerized polyamide thin-film composite membranes for dehydrating tetrahydrofuran. J. Membr. Sci. 2014, 470, 411–420. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Liao, Y.-L.; Sun, Y.-M.; Hu, C.-C.; Lai, J.-Y.; Liu, Y.-L. Lignin as an effective agent for increasing the separation performance of crosslinked polybenzoxazine based membranes in pervaporation dehydration application. J. Membr. Sci. 2019, 578, 156–162. [Google Scholar] [CrossRef]









| Membrane | Content of Carbon Nanoparticles, wt.% | Cross-Linking Method | Type and Number of PEL Bilayers |
|---|---|---|---|
| SA/UPM | - | - | - |
| SA/RC | - | - | - |
| SA/PAN | - | - | - |
| SA-5HF/PAN | 5% C60(OH)22–24 | - | - |
| SA-5AF/PAN | 5% C60-Arg | - | - |
| SA/PANCaCl2 | - | 1.25 wt.% CaCl2 | - |
| SA-5HF/PANCaCl2 | 5% C60(OH)22–24 | 1.25 wt.% CaCl2 | - |
| SA-5AF/PANCaCl2 | 5% C60-Arg | 1.25 wt.% CaCl2 | - |
| SA/PANCaCl2–5LblPSS/CS | - | 1.25 wt.% CaCl2 | Five bilayers of PSS/CS |
| SA/PANCaCl2–5LblPSS/PAH | - | 1.25 wt.% CaCl2 | Five bilayers of PSS/PAH |
| SA/PANCaCl2–5LblPSS/SA | - | 1.25 wt.% CaCl2 | Five bilayers of PSS/SA |
| SA/PANCaCl2–5LblPAA/SA | - | 1.25 wt.% CaCl2 | Five bilayers of PAA/SA |
| SA-5HF/PANCaCl2–5LblPSS/CS | 5% C60(OH)22–24 | 1.25 wt.% CaCl2 | Five bilayers of PSS/CS |
| SA-5AF/PANCaCl2–5LblPSS/CS | 5% C60-Arg | 1.25 wt.% CaCl2 | Five bilayers of PSS/CS |
| Membrane | Permeation Flux, kg/(m2 h) | Water Content in the Permeate, wt.% | Separation Factor (β) | PSI, kg/(m2 h) |
|---|---|---|---|---|
| SA | 0.151 | >99.9 | 73,326 | 11,072 |
| SA/UPM | 0.173 | 98.7 | 536 | 93 |
| SA/RC | 0.200 | 99.4 | 1215 | 243 |
| SA/PAN | 0.266 | >99.9 | 73,326 | 19,504 |
| Membrane | Ra, nm | Rq, nm |
|---|---|---|
| SA/PAN | 0.7 | 0.9 |
| SA-5HF/PAN | 3.5 | 4.9 |
| SA-5AF/PAN | 10.5 | 14.9 |
| Substrate | Ra, nm | Rq, nm | Total Porosity, % | Water Flux at 1 bar, L/(m2 h) |
|---|---|---|---|---|
| UPM | 22.4 | 28.7 | 95.0 | 60 |
| RC | 21.3 | 25.4 | 87.4 | 200 |
| PAN | 26.5 | 38.8 | 96.5 | 450 |
| Membrane | Ra, nm | Rq, nm |
|---|---|---|
| SA/PANCaCl2-5LblPSS/SA | 1.4 | 1.8 |
| SA-5HF/PANCaCl2-5LblPSS/SA | 2.4 | 3.2 |
| SA-5AF/PANCaCl2-5LblPSS/SA | 5.9 | 9.8 |
| Membranes | Water Content in Feed, wt.% | Temperature, °C | Permeation Flux, kg/(m2 h) | Water Content in Permeate, wt.% | Separation Factor, (β) | Reference |
| SA-5HF/PANCaCl2–5LblPSS/SA | 20 | 22 | 0.765 | >99.9 | 39,996 | This study |
| PVA–PAH (4.7%)/PAN–LblPSS,PAH (ten bilayers) | 20 | 20 | 0.061 | 99.9 | 3996 | [27] |
| PVA–PAH (4.7%)/UPM–LblPSS,PAH (ten bilayers) | 20 | 20 | 0.261 | 68.4 | 9 | |
| PVA-fullerenol (5%)-CS (20%)/UPM–LblPSS,CS (five bilayers) | 20 | 22 | 0.340 | 95.6 | 87 | [40] |
| PVA-fullerenol (5%)-CS (20%)/UPM–LblPSS,PAH (five bilayers) | 20 | 22 | 0.282 | 95.5 | 85 | |
| PVA-fullerenol(5%)-PAH (4.7%)/UPM–LblPSS,PAH (ten bilayers) | 20 | 22 | 0.286 | 98.4 | 246 | [41] |
| HEC */SA-fullerenol (5%)/PANCaCl2–LblPSS,PAH (five bilayers) | 20 | 22 | 0.976 | 92.8 | 52 | [70] |
| HEC */SA-fullerenol (5%)/PANCaCl2–LblPSS,SA (five bilayers) | 20 | 22 | 0.867 | 97.5 | 156 |
| Membranes | Membrane Type | Water Content in Feed, wt.% | Temperature, °C | Permeation Flux, kg/(m2 h) | Separation Factor, (β) | Reference |
|---|---|---|---|---|---|---|
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 12 | 22 | 0.681 | 73,326 | This study |
| SA-chitosan wrapped MWCNT (2%) | dense | 10 | 30 | 0.218 | 6419 | [71] |
| SA-phosphomolybdic acid (10%) | dense | 10 | 30 | 0.282 | 9028 | [72] |
| SA-phosphotungstic acid modified by ammonium carbonate (10%) | dense | 10 | 30 | 0.316 | 8991 | [73] |
| SA-gelatin (10%) | dense | 10 | 30 | 0.085 | 4277 | [74] |
| SA-fullerenol (5%) CaCl2 | dense | 12 | 22 | 0.240 | 73,326 | [47] |
| SA-fullerenol (5%)/PANCaCl2 | supported | 12 | 22 | 0.641 | 73,326 | |
| PERVAPTM 1201 | supported | 12 | 22 | 0.028 | 73,326 | |
| HEC */SA-fullerenol (5%)/PANCaCl2 | supported | 12 | 22 | 0.420 | 73,326 | [70] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 30 | 22 | 1.090 | 23,331 | This study |
| SA-poly(acrylamide) grafted guar gum (75/25) | dense | 30 | 30 | 0.164 | 153 | [75] |
| SA-polystyrene sulfonic acid-co-maleic acid | dense | 30 | 30 | ~0.223 | ~1800 | [76] |
| SA-aluminum with mesoporous silica (20%) | dense | 30 | 30 | 0.256 | ∞ | [77] |
| SA-heteropolyacids (10%) | dense | 30 | 30 | ~0.263 | ~1260 | [78] |
| SA-karayagum (15%) | dense | 30 | 30 | 0.486 | 1613 | [79] |
| SA-fullerenol (5%) CaCl2 | dense | 30 | 22 | 0.504 | 11,763 | [47] |
| SA-fullerenol (5%)/PANCaCl2 | supported | 30 | 22 | 1.202 | 2331 | |
| HEC */SA-fullerenol (5%)/PANCaCl2 | supported | 30 | 22 | 1.212 | 50 | [70] |
| SA-NGQD * (100 ppm)/PES * CaCl2 | supported | 30 | 25 | 1.822 | 788 | [80] |
| SA-OGQD * (100 ppm)/PES * CaCl2 | supported | 30 | 25 | 1.663 | 2331 | |
| SA-reduced graphene oxide (3%)/PES * CaCl2 | supported | 30 | 25 | ~1.750 | ~23,000 | [81] |
| SA-graphene quantum dots+reduced graphene oxide (3%)/PES * CaCl2 | supported | 30 | 25 | ~1.400 | ~23,000 |
| Membranes | Membrane Type | Water Content in Feed, wt.% | Temperature, °C | Permeation Flux, kg/(m2 h) | Separation Factor, (β) | Reference |
|---|---|---|---|---|---|---|
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 4 | 22 | 0.185 | 239,760 | This study |
| PVA/PS * hollow fiber membrane | supported | 5 | 50 | 0.06 | 53 | [82] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 12 | 22 | 0.568 | 73,326 | This study |
| Polyacrylic acid sodium-NaA zeolite/PAN | supported | 10 | 30 | 0.533 | 436 | [83] |
| DETA-TMC */CA * | supported | 10 | 25 | 0.860 | 1116 | [84] |
| DAPL-SCC */mPAN | supported | 10 | 25 | 0.600 | 264 | [85] |
| PAA-PA/PAN | supported | 10 | 25 | 0.830 | 1791 | [86] |
| PA-nanoNaX zeolite/mPAN | supported | 10 | 25 | 4.500 | 30 | [87] |
| 30 bilayers of CS/graphene oxide on mPAN | supported | 10 | 70 | 2.350 | 3390 | [88] |
| SA/PFSA */ceramic | supported | 15 | 75 | 0.821 | 5661 | [89] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 20 | 22 | 0.785 | 39,996 | This study |
| PVA-maleic acid/PES | supported | 20 | 60 | 0.444 | 13 | [90] |
| CS-PVA/PAN | supported | 20 | 60 | 1.500 | 40 | [91] |
| PVA-zeolite 4A (20%)/PAN | supported | 23.57 | 60 | 0.936 | 710 | [92] |
| Membranes | Membrane Type | Water Content in Feed, wt.% | Temperature, °C | Permeation Flux, kg/(m2 h) | Separation Factor (β) | Reference |
|---|---|---|---|---|---|---|
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 5.7 | 22 | 0.510 | 1086 | This study |
| PVA-HEC | dense | 5.5 | 30 | 0.082 | 160 | [93] |
| PVA-HEC *-clay microfiller | dense | 5.5 | 30 | 0.090 | 185 | |
| PVA-HEC *-clay nanofiller | dense | 5.5 | 30 | 0.112 | 195 | |
| CS-NaY zeolite | dense | 5 | 30 | 0.170 | 2092 | [94] |
| Polyaniline | dense | 4 | 55 | 0.622 | 36 | [95] |
| PVA-fullerenol (5%)/UPM | supported | 5.7 | 30 | 0.250 | 2347 | [96] |
| CS-polyacrylonitrile/UPM | supported | 5.7 | 35 | 0.202 | 1487 | [97] |
| CS-polystyrene/UPM | supported | 5.7 | 35 | 0.226 | 101 | |
| CMC-VP-31 (CM Celfa) | supported | 4 | 25 | 3.500 | 1976 | [98] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 12 | 22 | 0.807 | 342 | This study |
| BAE *-TMC/PAN | supported | 10 | 30 | 1.399 | 2036 | [99] |
| DAPE *-TMC/PAN | supported | 10 | 25 | 1.070 | 8991 | [100] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 20 | 22 | 0.873 | 96 | This study |
| crosslinked polybenzoxazine (CRPBz)-lignin | dense | 20 | 25 | 0.425 | 3996 | [101] |
| PVA-fullerenol (5%)/UPM | supported | 20 | 30 | ~1.000 | ~9 | [96] |
| SA-5HF/PANCaCl2-5LblPSS/SA | supported | 30 | 22 | 0.902 | 29 | This study |
| crosslinked polybenzoxazine (CRPBz)-lignin | dense | 30 | 25 | 0.490 | 19,440 | [101] |
| PVA-fullerenol (5%)/UPM | supported | 30 | 30 | ~1.200 | ~3.5 | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Lahderanta, E.; Solovyev, N.; Penkova, A. Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes. Membranes 2021, 11, 255. https://doi.org/10.3390/membranes11040255
Dmitrenko M, Liamin V, Kuzminova A, Lahderanta E, Solovyev N, Penkova A. Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes. Membranes. 2021; 11(4):255. https://doi.org/10.3390/membranes11040255
Chicago/Turabian StyleDmitrenko, Mariia, Vladislav Liamin, Anna Kuzminova, Erkki Lahderanta, Nikolay Solovyev, and Anastasia Penkova. 2021. "Modification Approaches to Enhance Dehydration Properties of Sodium Alginate-Based Pervaporation Membranes" Membranes 11, no. 4: 255. https://doi.org/10.3390/membranes11040255